Analysis of risk factors for and the prognosis of postoperative acute respiratory distress syndrome in patients with Stanford type A aortic dissection
Introduction
Acute respiratory distress syndrome (ARDS) can occur in cases of severe infection, shock, trauma, burns and other diseases following the damage to pulmonary capillary endothelial cells and alveolar epithelial cells (1,2). Currently, most investigators believe that ischemia-reperfusion injury (IRI) and the release of massive amounts of inflammatory mediators during cardiopulmonary bypass (CPB) and circulatory arrest are responsible for ARDS after cardiac surgery (3-6).
Most cases of Stanford type A aortic dissection (AD) require emergency surgery. Due to the complicated surgical procedures involved in the reconstruction of the aorta and its branches, CPB in addition to deep hypothermic circulatory arrest (DHCA) are essential for maintaining a relatively bloodless operating field. The difficulties and risks associated with these surgical procedures are therefore much greater than those of other types of AD (7).
However, few reports have elaborated on relationship between the ARDS and the surgery for Stanford type A AD. The goal of this study was to perform a retrospective analysis evaluating the risk factors and adverse consequences of postoperative ARDS in patients with Stanford type A AD who underwent thoracotomy surgeries in our department.
Methods
Clinical data
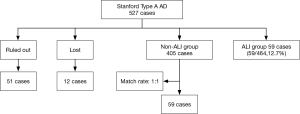
This retrospective nested case-control study included 527 patients who underwent surgeries for Stanford type A AD in the Department of Cardiovascular Surgery, Union Hospital, Fujian Medical University, between January 2013 and January 2016. These patients were divided into two groups: the ARDS group (n=59) and the non-ARDS group (n=405), Additionally, 59 patients in the non-ARDS group were randomly selected to serve as the matched controls. Sixty-three patients were excluded from the study according to the exclusion criteria, which are described in detail in Figure 1. The general demographic characteristics, underlying diseases, etiologies, intraoperative and postoperative conditions, recent and short-term complications, and hospitalization costs were accounted for in the analysis.
Diagnostic criteria for ARDS
Postoperative ARDS is defined as an oxygenation index (OI; PaO2/FiO2 <300) that occurs within 7 days after surgery and that is unrelated to other documented causes of acute lung injury (e.g., cardiogenic pulmonary edema, pneumonia, pulmonary embolism and hemato- or pneumothorax). ARDS patients routinely undergo chest radiography, pleural ultrasonography, bronchoscopy with bronchoalveolar lavage (BAL) and chest computed tomography to exclude other causes of postoperative respiratory failure. Perioperative heart failure occurring before the diagnosis of postoperative ARDS was ruled out as a possible cause of pulmonary dysfunction via the performance of transesophageal echocardiography assessing left ventricular systolic and diastolic function, and a pulmonary artery catheter was used to assess the pulmonary capillary wedge pressure (<18 mmHg) when necessary (8).
Exclusion criteria
- Patients with severe heart failure (NYHA classification level ≥ III grade) that was recognized before the AD surgery or the diagnosis of ARDS, including acute myocardial infarction, cardiac tamponade, severe primary aortic regurgitation, congenital heart disease, or valvular heart disease;
- Patients with other serious lung disease that was recognized before the AD surgery or the diagnosis of ARDS, including silicosis, pneumoconiosis, chronic obstructive pulmonary disease (COPD), lung cancer, and interstitial pneumonia;
- Patients and/or their relatives who did not agree to participate in this clinical experimental study.
Treatment
Once the diagnosis of ARDS had been confirmed, a protective strategy employing low tidal volume ventilation (6–8 mL/kg) was initiated. Moreover, large doses of ambroxol hydrochloride (90 mg iv bid), methylprednisolone (1 mg/kg iv bid) and ulinastatin (5,000 units/kg iv bid) were injected to eliminate the effects of inflammatory mediators. Continuous renal replacement therapy (CRRT) was implemented in patients with renal insufficiency (9). Cardiac and diuretic treatments, regular anti-infection strategies, an intra-aortic balloon pump (IABP) and/or an extracorporeal membrane oxygenator (ECMO) were employed in cases of heart failure or serious pulmonary dysfunction.
Evaluation of the functions of the lung and other organs
Pulmonary function was evaluated based on the OI according to perioperative arterial blood gas analysis, and the checkpoints were defined as the perioperative time point (T0) and 12, 24, 48, and 72 hours after DHCA (T1, T2, T3, and T4, respectively).
The health statuses of the patients were evaluated according to the criteria of the acute physiology and chronic health evaluation II (APACHE II), which includes age, rectal temperature, mean arterial pressure, heart rate, respiratory rate, pH of the arterial blood pH, venous blood bicarbonate, sodium and potassium levels in the venous blood, serum creatinine level, hematocrit, peripheral leukocyte density, Glasgow score, and chronic underlying disease. The checkpoints were identical to those applied for assessment of the OI.
Postoperative follow-up
The doctors maintained telephone contact with the patients after discharge. Periodic reviews were performed every three to six months to assess the clinical signs and symptoms, vital signs, echocardiograms, and CTAs of the thoracic and abdominal aorta. The follow-up reviews were conducted each year beginning 12 months after discharged. In cases of death after discharge, the cause was ascertained with the relatives’ permission.
Ethics
The Ethics Committees of Fujian Medical University Union Hospital approved this retrospective study.
Statistical analysis
SPSS 16.0 software was used for the statistical analyses. Descriptive statistical analyses, repeated-measures ANOVAs and Wilcoxon rank sum tests were used to analyze measurement data. The chi-square test, Fisher’s exact test and multivariate logistic regression analysis were used to analyze the count data. The Kaplan-Meier method was used to plot the survival curves. Statistical significance was defined as P<0.05.
Results
General clinical data
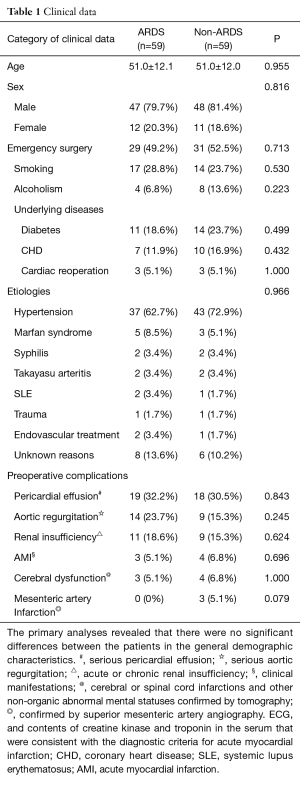
In total, 464 patients with Stanford type A AD who were treated with thoracotomy were included in our retrospective study after excluding 51 patients and 12 lost patients. Fifty-nine of the included patients (59/464, 12.7%) experienced postoperative ARDS and were matched with 59 patients from the non-ARDS group. The majority of the ARDS patients were diagnosed within 72 hours after AD surgery. The primary analyses revealed that there were no significant differences between the patients in terms of age, gender, emergency surgery, smoking, alcoholism, underlying disease, preoperative complications, or etiology (Table 1).
Full table
Surgical and perioperative treatments
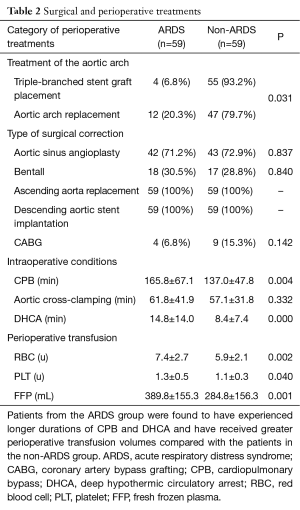
All of the patients were divided into two groups according to the treatment of the aortic arch [i.e., open placement of a triple-branched stent graft (10) or replacement of the entire aortic arch (11)]. A Fisher’s exact test revealed that the type of surgical correction had no effect on the incidence of postoperative ARDS. However, a Pearson’s chi-square test revealed that the patients who underwent open placement of a triple-branched stent graft exhibited a lower incidence of postoperative ARDS (6.8% vs. 20.3%, P=0.031) (Table 2). We also discovered that the patients in the postoperative ARDS group experienced significantly longer durations of CBP (165.8±67.1 vs. 137.0±47.8 min, P=0.004) and DHCA (14.8±14.0 vs. 8.4±7.4 min, P=0.000) and received much larger perioperative transfusion volumes of RBCs (7.4±2.7 vs. 5.9±2.1 u, P=0.001), PLTs (1.3±0.5 vs. 1.1±0.3 u, P=0.040) and FFPs (389.8±155.3 vs. 284.8±156.3 mL, P=0.001) than the patients in the non-ARDS group. However, no significant difference in the aortic clamping time was observed between the two groups (Table 2).
Full table
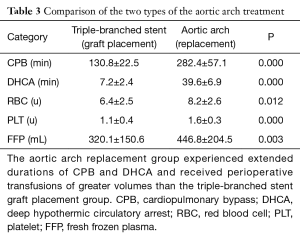
Moreover, a chi-square test indicated that the durations of CBP (282.4±57.1 vs. 130.8±22.5 min, P=0.000) and DHCA (39.6±6.9 vs. 7.1±2.3 min, P=0.000) were significantly longer for the group of patient receiving total aortic arch replacement than for the group of patients receiving triple-branched stent engraftment, and a significant difference in the perioperative transfusion volume was found between these two groups (Table 3).
Full table
Postoperative outcomes
The postoperative oxygenation index (OI) values was lower in the patients in the ARDS group than in the patients in the non-ARDS group (F=21.280, P=0.000). Moreover, the postoperative Apache II scores of the patients in the ARDS group were higher than those of the patients in the non-ARDS group (F=25.918, P=0.000) (Figures 2,3). These findings revealed that ARDS had seriously adverse effects on postoperative oxygenation and physiological function.
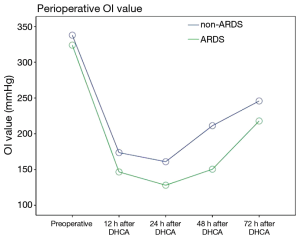
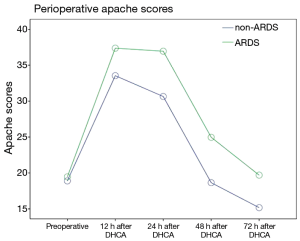
The observed postoperative complications included reoperation (debridement or thoracotomy for hemostasis), heart dysfunction that occurred after the diagnosis of ARDS, central nervous system (CNS) complications, renal insufficiency, pulmonary infection that occurred after the diagnosis of ARDS, wound infection, liver dysfunction, and multiple organ dysfunction syndrome (MODS). A total of 8 in-hospital deaths among the 118 patients (6.8%) occurred due to MODS. The remaining patients survived and were successfully discharged with a normal OI. However, the incidences of MODS (16.9% vs. 5.1%, P=0.040, OR=4.278; 95% CI: 1.127–16.232), lung infection (22.0% vs. 6.8%, P=0.018, OR=3.886; 95% CI: 1.186–12.736) and in-hospital mortality (11.9% vs. 1.7%, P=0.028, OR=7.808, 95% CI: 0.929–65.600) were significantly higher among the patients in the ARDS group than among the patients in the non-ARDS group (Table 4), and the ARDS group exhibited significantly longer durations of ventilation and ICU stay. Furthermore, the hospital costs incurred by the patients in the ARDS group were substantially higher than those incurred by the non-ARDS patients (164,727.9±14,904.1 vs. 126,827.6±11,635.0 RMB, P=0.000), and this difference was primarily due to the postoperative complications associated with ARDS (Table 4).
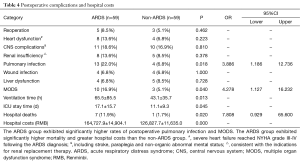
Full table
The non-survivors with ARDS were found to experience extended durations of CPB (233.9±60.9 vs. 156.6±62.9 min, P=0.003), aortic cross-clamping (113.6±63.5 vs. 54.8±33.1 min, P=0.012) and DHCA (35.7±17.6 vs. 12.0±10.9 min, P=0.000) and receive much larger perioperative transfusion volumes of FFPs (514.3±149.2 vs. 367.1±149.7 mL, P=0.009) than the survivors with ARDS (Table 5).
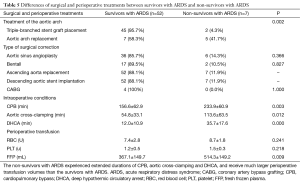
Full table
Follow-up
Twelve patients were lost within the 36-month follow-up period and were not included in this study. Four incidents of death after discharge were observed and were attributed to car accident, stroke, recrudescence of the AD and unknown cause (Table 6). Short-term complications included recrudescence of the AD (1 patient died and 2 patients survived with no significant sequelae after emergency reoperation), endoleaks (3 cases that resolved in 1 to 3 months, 4 cases that were treated with endovascular stent grafts, and 1 case that was treated with a triple-branched stent graft; all 8 patients ultimately recovered), paraplegia and infection (the body temperatures and hemograms returned to normal after 12 to 21 days of anti-infection treatment). Additional chi-square tests indicated that there were no significant differences between the two groups in terms of mortality or complications after discharge (Table 6).

Full table
Multivariate logistic regression analysis
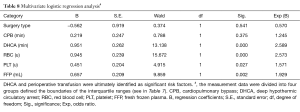
A total of 6 variables were analyzed in the stepwise logistic regression model to identify the independent risk factors for postoperative ARDS. DHCA and perioperative transfusion were ultimately identified as significant risk factors (Tables 7,8).

Full table
Full table
Survival curves
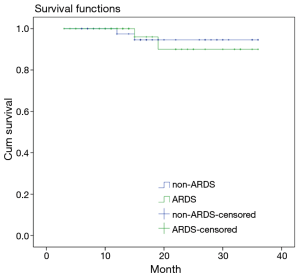
Survival curve analyses based on 36 months of observation revealed that no significant difference in the survival rate or the median survival time between the patients in the ARDS group and the patients in the non-ARDS group (Figure 4).
Discussion
A temporary block of the systemic circulation is required to create a relatively bloodless surgical field during treatment of Stanford type A AD treatment. Despite the application of improved surgical procedures and protective techniques such as selective cerebral perfusion and DHCA, the risk of postoperative organ dysfunction or organ failure persists. It has been reported that the frequency of ARDS following Stanford type A AD surgery is between 12.7% and 13.0% (12-15), which is similar to the 12.7% (59/464) incidence observed in our department.
In the absence of effective treatment, the mortality rate of ARDS patients over the past two decades has fluctuated between 25% and 67%. In the present study, we observed that a total of 7 of the 8 (87.5%) in-hospital deaths were associated with ARDS. Moreover, a total of 7 of the 59 (11.9%) patients with postoperative ARDS died in the hospital despite receiving adequate treatment.
In addition to impairing pulmonary oxygenation and causing refractory hypoxemia, ARDS has serious irreversible effects on other vital organs and can lead to MODS. The mortality rate of MODS has been reported to be as high as 53.3% (16). Additionally, significantly lower OI and higher Apache II scores were observed in the patients in the ARDS group than in the patients in the non-ARDS group. These statistics explain why the proportion of patients with postoperative MODS was significantly higher in the ARDS group than in the non-ARDS group. This phenomenon indicated that the high mortality rate of postoperative MODS has a serious influence on the prognoses of patients with postoperative ARDS. Additionally, once postoperative ARDS occurred, the durations of ventilation and ICU stay lengthened significantly, which increased the incidences of ventilator-associated pneumonia (VAP), nosocomial infections and other complications in addition to increasing the economic burdens on the patients. Furthermore, the survival rate of the patients in the ARDS group after 36 months of follow-up was lower than that of the non-ARDS group. Preventing postoperative ARDS is therefore particularly important for patients with Stanford type A AD.
The most common causes of postoperative ARDS are pulmonary injury due to IRI resulting from DHCA during surgical treatment of Stanford type A AD treatment, cytokine-mediated inflammation during CPB, and leukocyte infiltration into the pulmonary tissue. ARDS can also result in systemic inflammatory response syndrome (SIRS) and IRI of other organs, such as the intestines (17,18), skeletal muscle (19), and liver (20). These factors account for the significantly longer DHCA and CPB times of the ARDS group compared with those of the non-ARDS group, Further, the results of a subsequent multivariate logistic regression analysis indicated that DHCA was an independent risk factor for postoperative ARDS. Thus, these results demonstrated that recipients of treatment for Stanford type A AD would benefit from reductions in the CPB and DHCA times and the mitigation of the IRI resulting from improved surgical techniques.
Transfusion-related acute lung injury (TRALI) also plays an important role in postoperative ARDS following Stanford type A AD and has been attributed to alveolar epithelial cell and endothelial cell damage by active neutrophils. The molecular mechanism underlying this phenomenon is unclear but is thought to involve the S100A12 protein (21,22) or antigen-antibody reactions. The surgical treatment of Stanford type A AD is often complicated and may involve vascular anastomosis, which requires the transfusion of a greater amount of blood products than conventional thoracotomy. This theory is consistent with our findings that the amounts of perioperative transfusions (regardless of the type of blood product) were significantly greater in the ARDS group than in the non-ARDS group. Further logistic regression prompted us to conclude that perioperative transfusion was an independent risk factor for postoperative ARDS. Thus, surgical treatment of Stanford type A AD was directly associated with TRALI. Therefore, reducing the use of blood products by improving operative techniques is critical for the prevention of postoperative ARDS and reducing the incidence of in-hospital death with ARDS in patients with Stanford type A AD patients.
Interestingly, the present study demonstrated that 20.3% of the patients with ARDS had receive replacement of the entire aortic arch (Sun’s procedure), and the corresponding percentage in the non-ARDS group was only 6.8%. This difference can be explained by the longer durations of DHCA and CPB and the much greater perioperative blood transfusions associated with Sun’s procedure. Because we had demonstrated that the duration of DHCA and the amount of perioperative blood transfusion were independent risk factors for postoperative ARDS, we speculate that open placement of a triple-branched stent graft is more suitable for Stanford A type AD and that implementing this procedure could effectively reduce the incidence of postoperative ARDS.
However, although the perioperative blood transfusion volumes required by the recipients of triple-branched stent engraftment were significantly less than the volumes required by the recipients of aortic arch replacement, and although the latter patients required a much longer duration of DHCA, the type of aortic arch treatment was not an independent risk factor for postoperative ARDS. Possible explanations for this observation include the following: (I) the mechanism of postoperative ARDS in patients with Stanford type A AD involves a variety of pathophysiological process; and (II) the direct pulmonary injury caused by Stanford type A AD could play an important role in ARDS.
Conclusions
ARDS results from multiple factors and has a serious influence on the prognoses of patients following surgical treatment of Stanford type A AD, and postoperative ARDS warrants greater attention by cardiac surgeons. Controlling the volume of perioperative RBC transfusions required may reduce the incidence of postoperative ARDS, and reducing the duration of CPB and DHCA times will benefits the recipients of thoracic surgeries. Open placement of a triple-branched stent graft is a more suitable treatment for Stanford A type AD and effectively reduced the incidence of postoperative ARDS.
Acknowledgments
The authors thank the patients who participated in the study and the research assistants and study coordinators who assisted with the data collection and management of the study, including Hua Cao, Sheng Chen, Qian-Zhen Li, Liang-Liang Yan, Yong Lin, Yun-Nan Hu, Lin Lu, Ling-Fen Li and Jia-Xing Zhang.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Ethics Committees of Fujian Medical University Union Hospital approved this retrospective study and written informed consent was obtained from all patients.
References
- Pierrakos C, Karanikolas M, Scolletta S, et al. Acute respiratory distress syndrome: pathophysiology and therapeutic options. J Clin Med Res 2012;4:7-16. [PubMed]
- Müller-Redetzky HC, Felten M, Hellwig K, et al. Increasing the inspiratory time and I:E ratio during mechanical ventilation aggravates ventilator-induced lung injury in mice. Crit Care 2015;19:23. [Crossref] [PubMed]
- Makita S, Ohira A, Tachieda R, et al. Behavior of C-reactive protein levels in medically treated aortic dissection and intramural hematoma. Am J Cardiol 2000;86:242-4. [Crossref] [PubMed]
- Morimoto N, Morimoto K, Morimoto Y, et al. Sivelestat attenuates postoperative pulmonary dysfunction after total arch replacement under deep hypothermia. Eur J Cardiothorac Surg 2008;34:798-804. [Crossref] [PubMed]
- Warren OJ, Smith AJ, Alexiou C, et al. The inflammatory response to cardiopulmonary bypass: part 1--mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009;23:223-31. [Crossref] [PubMed]
- Mortelliti MP, Manning HL. Acute respiratory distress syndrome. Am Fam Physician 2002;65:1823-30. [PubMed]
- Liu ZG, Sun LZ, Chang Q, et al. Should the "elephant trunk" be skeletonized? Total arch replacement combined with stented elephant trunk implantation for Stanford type A aortic dissection. J Thorac Cardiovasc Surg 2006;131:107-13. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Mehta RL. Indications for dialysis in the ICU: renal replacement vs. renal support. Blood Purif 2001;19:227-32. [Crossref] [PubMed]
- Chen LW, Dai XF, Zhang GC, et al. Total aortic arch reconstruction with open placement of triple-branched stent graft for acute type A dissection. J Thorac Cardiovasc Surg 2010;139:1654-1655.e1. [Crossref] [PubMed]
- Ma WG, Zhu JM, Zheng J, et al. Sun's procedure for complex aortic arch repair: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:642-8. [PubMed]
- Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 2007;369:1553-64. [Crossref] [PubMed]
- Fanelli V, Vlachou A, Ghannadian S, et al. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis 2013;5:326-34. [PubMed]
- Milberg JA, Davis DR, Steinberg KP, et al. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983-1993. JAMA 1995;273:306-9. [Crossref] [PubMed]
- Reynolds HN, McCunn M, Borg U, et al. Acute respiratory distress syndrome: estimated incidence and mortality rate in a 5 million-person population base. Crit Care 1998;2:29-34. [Crossref] [PubMed]
- Ge QG, Hou J, Zhou H, et al. Multiple organ failure induced by acute respiration distress syndrome: a report of 15 cases. Mod Surg 2000;6:41-4.
- He X, Han B, Mura M, et al. Anti-human tissue factor antibody ameliorated intestinal ischemia reperfusion-induced acute lung injury in human tissue factor knock-in mice. PLoS One 2008;3:e1527. [Crossref] [PubMed]
- Hei ZQ, Gan XL, Huang HQ, et al. Protective effects of cromolyn sodium on intestinal ischaemia-reperfusion-triggered lung injury in rats. Br J Anaesth 2007;98:407-8. [Crossref] [PubMed]
- Harkin DW, Arnold R, Hoper M. Anti-endotoxin hyperimmune globulin attenuates portal cytokinaemia, phagocytic cell priming, and acute lung injury after lower limb ischaemia-reperfusion injury. Eur J Vasc Endovasc Surg 2007;33:330-9. [Crossref] [PubMed]
- Cao DQ, Chen YP, Chang YT, et al. Effects of aminoguanidine on the lung injury induced by the total hepatic ischemia-reperfusion in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2006;31:94-6. [PubMed]
- Müller MC, Tuinman PR, Vlaar AP, et al. Contribution of damage-associated molecular patterns to transfusion-related acute lung injury in cardiac surgery. Blood Transfus 2014;12:368-75. [PubMed]
- Kikkawa T, Sato N, Kojika M, et al. Significance of measuring S100A12 and sRAGE in the serum of sepsis patients with postoperative acute lung injury. Dig Surg 2010;27:307-12. [Crossref] [PubMed]


