How to deal with subcentimeter lung cancer: a moving target!
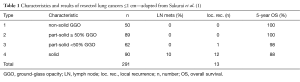
In the manuscript “Clinicopathologic features of resected subcentimeter lung cancer” published in the Annals Of Thoracic Surgery 2015;99:1731-8, H. Sakurai and colleagues from the Division of Thoracic Surgery, National Cancer Center Hospital, and Department of General Thoracic Surgery, Keio University Graduate School of Medicine, Tokyo, Japan, describe 291 patients who underwent resection of a subcentimeter lung cancer (1). Tumors were subdivided into four categories depending on the percentage of ground-glass opacity (GGO) ranging from 100% GGO (non-solid lesions) to 0% GGO (solid lesions) with part-solid lesions in between. The characteristics of the four groups and results of their study are presented in Table 1. Clinically, all tumors were stage IA. A variety of resections was performed with an increasing frequency of lobectomies from group 1 till 4. Conversely, sublobar resections were the most common procedure in groups 1 and 2. Most of these patients underwent wedge resection with anatomical segmentectomy being less frequently performed.
Full table
Adenocarcinoma was the most common histological diagnosis with non-invasive bronchioloalveolar carcinoma (BAC) found in 84% of type 1 tumors, and in only 8% in type 4 tumors. Interestingly, one patient in group 4 had an incidentally discovered small cell lung cancer. Lymph node metastases were exclusively encountered in group 4 (10%). Recurrent disease was noted in 13 patients, 1 belonging to group 3 and 12 to group 4. Overall and disease-free survival rates were excellent for types 1-3 but a significantly lower survival was noted for type 4 (Table 1). The authors conclude that limited resection may be considered in types 1-3, but lobectomy should still be the standard operative procedure for type 4 tumors (1).
This interesting study sheds new light on diagnosis and management of early lung cancers and focusses specifically on lesions ≤1 cm, clinically staged as IA disease. Although the conclusions are valid and clinically applicable, the study is retrospective and there is an inherent selection bias as only operated patients were considered. In this respect the current study represents a post-hoc analysis meaning that the results cannot be extrapolated to all patients with clinical T1a lesions ≤1 cm.
In the present study the majority of lesions were adenocarcinomas in all subgroups, even representing 100% in subgroups 1 and 2. A new adenocarcinoma classification was published in 2011 by a common task force of the International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS) and European Respiratory Society (ERS) (2). As new subcategories, adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) were introduced and different subsets of invasive adenocarcinoma including acinar, papillary, micropapillary and solid patterns, were defined allowing for a detailed histological assessment of lung cancers. In relation to this classification it should be noted that a solid nodule on chest computed tomography (CT) not necessarily corresponds to the specific histologic variant of solid adenocarcinoma. These two entities may be easily mixed up and a clear distinction between imaging and histological characteristics should be made.
The term bronchioloalveolar carcinoma (BAC) and mixed subtype adenocarcinoma are not used anymore in this new classification as they gave rise to much confusion and inappropriate description of these lung cancers (2). This new classification also has profound surgical implications for small, early-stage lung cancers mainly in relation to sublobar resection including anatomical segmentectomy and wedge resection, and recommended lymph node dissection (3). In the updated 2015 World Health Organisation (WHO) histological classification of tumors of the lung, pleura, thymus and heart this 2011 adenocarcinoma classification is incorporated without any changes (4,5).
Unfortunately, in the present report the term BAC is still used and the large group of adenocarcinomas was not further subdivided into specific subsets as defined in the 2011 classification (2). So, no specific conclusions can be made regarding the type of resection in correlation with a specific histological diagnosis.
At the present time it is clear that adenocarcinomas with solid or micropapillary growth patterns have the poorest prognosis (6,7). Most probably, with the latter histological diagnoses a lobectomy is still the most appropriate treatment to obtain good long-term results. A newly recognized pattern is spread through air spaces (STAS) where tumor deposits are present in lung parenchyma around the primary tumor without direct connection (8). As can be expected, a higher rate of recurrences has been described with this particular growth pattern (8).
Besides histology also size matters and this is recognized in the 8th edition of the TNM classification where the T1 subset will be subdivided into 3 categories with 1 cm cut-off values: T1a will include tumors ≤1 cm, T1b tumors between 1.1–2.0 cm and T1c tumors between 2.1 and 3.0 cm (9). From the large IASLC database it is clear that the smaller tumours have the best prognosis with a 5-year estimated survival rate for clinically and pathologically staged non-small cell lung cancers (NSCLC) ≤1 cm without lymph node or distant metastases, of 92% and 91%, respectively.
Another important change is that for determining the T descriptor only the invasive part will be considered although it is recommended to also provide the total tumor size in the pathology report (10).
As the natural history of pulmonary subsolid nodules including pure GGO and part-solid nodules, is rather elusive, a recent prospective study evaluated the natural course of these lesions (11). They were subdivided into pure ground-glass nodules (GGNs), heterogeneous GGNs with the solid component detected only in lung windows on chest CT, and part-solid nodules. A total of 1,229 subsolid nodules were included with a mean prospective follow-up period of 4.3 years. Among the 1,046 pure GGNs, 1.2% developed into heterogeneous GGNs and 5.4% into part-solid nodules. Among the 81 heterogeneous GGNs 19.8% developed into part-solid nodules. Regarding the pure GGNs the mean period until the development into part-solid nodules was 3.8 years, and for the heterogeneous GGNs 2.1 years. Invasive adenocarcinomas were diagnosed only among the part-solid nodules, corresponding to 1% of all 1,229 subsolid nodules (11).
When the results of the National Lung Screening Trial (NLST) became available demonstrating a clear survival advantage for screening with low-dose CT compared to chest radiography, many screening programmes were established and confirmatory studies are performed (12). The results of the European Nelson trial will be available at the end of 2016 (13). Due to this screening studies thoracic oncologists and surgeons have recently been confronted with the new challenge of histological diagnosis and management of small, screen-detected nodules (14). It has become clear that only a minority represent early invasive lung cancers. Many societies developed specific screening guidelines which are updated when new findings are published. Regarding surgical management the main objectives of the task force established by the Society of Thoracic Surgeons (STS) were optimizing management of screen-detected lung cancers and minimizing the morbidity of false-positive diagnoses (15). The STS task force believes that CT screening programs should follow a formal algorithmic approach to standardize management. Regarding surgical intervention the least parenchymal resection compatible with current diagnostic and oncological principles should be performed by the least invasive surgical approach. Moreover, the STS task force advocates standardized pathological reporting according to the criteria of the 2011 adenocarcinoma classification (2,15). As mentioned before, this 2011 classification was completely integrated in the 2015 WHO classification (4).
More recently, the European Society of Thoracic Surgeons (ESTS) also created a working group devoted to screening. Recommendations have been prepared that cover the essential aspects to be taken into account when considering implementation of CT screening in Europe. Specific topics considered are the implementation of CT screening in Europe, participation of thoracic surgeons in CT screening programs, training and clinical profile for surgeons participating in screening programs, the use of minimally invasive thoracic surgery, and other relevant issues. These recommendations will be published in the European Journal of Cardiothoracic Surgery in the near future (manuscript submitted, personal communication). Also, the Fleischner Society which previously published specific criteria for management of solid and subsolid nodules will publish updated recommendations at the end of 2016 (manuscript submitted, personal communication) (16,17).
Regarding small lung cancers the main controversy for thoracic surgeons centers around the indications and long-term results of sublobar resection and whether a wide wedge resection yields equivalent results to anatomical segmentectomy. Until now, only one large prospective randomized trial was fully published which was performed by the Lung Cancer Study Group in the USA and published in 1995 (18). In this study patients with stage I NSCLC were randomized between lobectomy and sublobar resection. The limited resection group had a three-fold increase in local recurrence (P=0.008), a 30% increase in overall death rate (P=0.08), and a 50% increase in cancer related death (P=0.09) compared to patients undergoing lobectomy. It should be noted that limited resections included anatomical segmentectomies as well as non-anatomical wedge resections, and that both T1a and T1b tumours were included. Although the survival results were borderline significant, lobectomy became the gold standard, even for early-stage lung cancer (18,19). Many retrospective studies mainly coming from Japan, showed that for small, early NSCLC sublobar resection may be an oncologically valid procedure, also for low risk patients who might tolerate a major lung resection. In a review of sublobar resection segmentectomy yielded similar results as lobectomy for tumors ≤2 cm (20). In a meta-analysis of sublobectomy versus lobectomy published in 2012 similar results for overall survival were found for stage IA NSCLC ≤2 cm with a hazard ratio of 0.81 (21). To obtain more high-grade evidence, two large randomized trials are undertaken comparing segmentectomy versus lobectomy for NSCLC ≤2 cm, the US trial CALGB 140503 and Japanese study JCOG0802/WJOG4607L (22,23). The Japanese study has finished accrual but final results are not available yet. In the meantime accepted criteria for sublobar resection include stage IA disease without nodal involvement, peripheral tumor location and a predominantly GGO appearance on chest CT scan (24). For all other lesions lobectomy remains the gold standard and every attempt should be made to obtain a complete R0 resection according to the IASLC criteria (25). Limited resections may also be considered appropriate in patients presenting a high risk for a major lung resection (26).
Another important issue in this regard is the accuracy of frozen section analysis to determine the extent of resection intraoperatively in low risk patients. In a recent review of 361 resected stage I adenocarcinomas ≤3 cm frozen section analysis had a high specificity but low sensitivity for micropapillary and solid patterns (27). In a retrospective study of 803 patients with stage I adenocarcinoma the concordance rate between frozen section analysis and final pathology was 84.4% (28). Most discrepancies consisted of underestimation of AIS and MIA. The accuracy of frozen section analysis was 90.8% for lesions >1 cm but only 79.6% for lesions ≤1 cm. The latter finding is rather troublesome and means that in some cases of subcentimeter lesions undergoing sublobar resection, a completion lobectomy may be indicated by a subsequent, second intervention when initial analysis showed a noninvasive or minimally invasive lung cancer but definitive pathological examination reveals an invasive cancer with micropapillary or solid pattern, especially when also spread through air spaces is discovered. Preoperatively patients should be clearly informed about this possible secondary procedure.
In conclusion, this important retrospective analysis provides interesting data on management of subcentimeter lung cancer although many questions remain unanswered (1). With the official introduction of the 8th TNM classification in January 2017 and further application of the 2015 WHO histological classification of lung cancer paying particular attention to early types of adenocarcinoma, further studies including screening trials, will provide more insight on specific diagnostic and therapeutic algorithms for this fascinating but challenging subgroup of patients with early-stage lung cancer.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Gang Shen, MMSC (The Second Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Sakurai H, Nakagawa K, Watanabe S, et al. Clinicopathologic features of resected subcentimeter lung cancer. Ann Thorac Surg 2015;99:1731-8. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. [Crossref] [PubMed]
- Van Schil PE, Asamura H, Rusch VW, et al. Surgical implications of the new IASLC/ATS/ERS adenocarcinoma classification. Eur Respir J 2012;39:478-86. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol 2015;10:1240-2. [Crossref] [PubMed]
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [Crossref] [PubMed]
- Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105:1212-20. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Kakinuma R, Noguchi M, Ashizawa K, et al. Natural History of Pulmonary Subsolid Nodules: A Prospective Multicenter Study. J Thorac Oncol 2016;11:1012-28. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17:907-16. [Crossref] [PubMed]
- Flores R, Bauer T, Aye R, et al. Balancing curability and unnecessary surgery in the context of computed tomography screening for lung cancer. J Thorac Cardiovasc Surg 2014;147:1619-26. [Crossref] [PubMed]
- Rocco G, Allen MS, Altorki NK, et al. Clinical statement on the role of the surgeon and surgical issues relating to computed tomography screening programs for lung cancer. Ann Thorac Surg 2013;96:357-60. [Crossref] [PubMed]
- MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395-400. [Crossref] [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Lederle FA. Lobectomy versus limited resection in T1 N0 lung cancer. Ann Thorac Surg 1996;62:1249-50. [Crossref] [PubMed]
- Rami-Porta R, Tsuboi M. Sublobar resection for lung cancer. Eur Respir J 2009;33:426-35. [Crossref] [PubMed]
- Fan J, Wang L, Jiang GN, et al. Sublobectomy versus lobectomy for stage I non-small-cell lung cancer, a meta-analysis of published studies. Ann Surg Oncol 2012;19:661-8. [Crossref] [PubMed]
- Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol 2010;5:1583-93. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Sihoe AD, Van Schil P. Non-small cell lung cancer: when to offer sublobar resection. Lung Cancer 2014;86:115-20. [Crossref] [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- De Waele M, Van Schil P. Limited resections in high-risk patients. Curr Opin Pulm Med 2015;21:309-13. [Crossref] [PubMed]
- Yeh YC, Nitadori J, Kadota K, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of ≤ 3 cm: accuracy and interobserver agreement. Histopathology 2015;66:922-38. [Crossref] [PubMed]
- Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol 2016;34:307-13. [Crossref] [PubMed]


