Stepwise approaches to optimize strategy for holding thoracoscope during single port video-assisted thoracoscopic surgery
Introduction
As an emerging technique, single port video-assisted thoracoscopic surgery (SP-VATS) was first reported by Rocco et al. in 2004 for dealing with simple thoracic procedures such as pneumothorax, pleural biopsy, and lung wedge resection, et al. (1). In 2011, Gonzalez et al. reported initial experience on SP-VATS lobectomy (2). Following that were numerous reports even on complicated cases of sleeve or even double-sleeve lobectomy within very short periods of time (3). Due to limited access it’s technically demanding for the surgeon during SP-VATS procedure, such as mutual interference among instruments, limited “work angle”, “arrow effect” among instruments, and lack of appropriate instruments. Coordination between the thoracoscope assistant and the surgeon was much more difficult. To provide good vision and enough operating room for the surgeon the thoracoscope assistant needed to hold the thoracoscope still in nearly distorted posture for hours, which was an exhausting work. In order to make the operation smoother and the assistant feel more comfortable during SP-VATS, we developed a strategy for holding the thoracoscope after stepwise approaches.
Surgical techniques
Preoperative assessment
The indications of SP-VATS procedure were similar to multi-portal VATS including mediastinal masses, pleural diseases, both benign and malignant pulmonary diseases, and so on. All patients underwent routine systemic function assessments, including blood tests and cardiopulmonary function text preoperatively. For cases with pulmonary malignancy, contrast enhanced computerized tomography (CT) scanning of the thorax and the upper abdomen, bone scintigraphy, magnetic resonance imaging of the brain, and bronchoscopy would be administered to patients with centrally located lesions.
Anesthesia, positioning, and incision
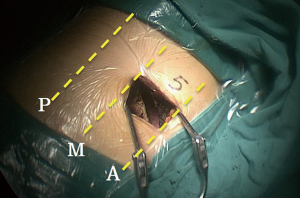
Written informed consent was obtained from each patient. All of the patients underwent general anesthesia through double-lumen endotracheal intubation. Appropriate single lung ventilation was accomplished before operation. Generally, each patient was placed in a folding knife gesture (with both of the cranial side and caudal side pushed down) in the lateral decubitus position. Both of the surgeon and the thoracoscope assistant stood in front of the patient (abdominal side) while the other assistant stood at the back side of the patient. There were monitors placed on both sides of the patient. A 3 to 5 cm incision was made in the 4th or 5th intercoastal space between the anterior and middle axillary lines (Figure 1).
Stepwise approaches
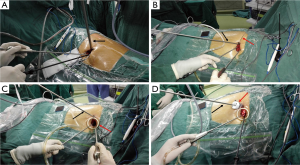
To explore an effective way for the thoracoscope assistant to better coordinate with the surgeon while not feeling labor exhausting when holding the thoracoscope, we’ve went through four stages.
Stage I (Figure 2A). A traditional 10-mm 30° thoracoscope, which was commonly used in multi-portal VATS procedure, was transferred into SP-VATS procedure. It was usually placed at the posterior part of the incision. The incision was spread by a retractor. There were several disadvantages of this strategy. The 10-mm scope was heavy but too short. The assistant should lift his hands holding it and step aside standing in a distorted posture to make room for the surgeon. In addition, it was easy to be displaced and vulnerable to be blurred when introduced into or out of the thoracic cavity.
Stage II (Figure 2B). The 10-mm thoracoscope was replaced by a lengthened 5-mm thoracoscope, which was lighter and longer. It was towed and fixed via a silk suture. Then the assistant didn’t need to maintain the position of the thoracoscope at the posterior part of the incision so hard. However, problems still existed. It’s not so convenient to be introduced into the thoracic cavity and also vulnerable to be blurred when introduced in or out of the thoracic cavity.
Stage III (Figure 2C). A soft plastic wound protector was placed to spread the incision. The 5-mm thoracoscope was placed outside of the wound protector at the posterior part of the incision. Thereafter the thoracoscope was more stable than that in stage II, and of course it would be labor saving for the assistant.
Stage IV (Figure 2D). The 5-mm thoracoscope (Karl Storz 26048BA 30 Degree Autoclavable Bariatric Laparoscope 5 mm) was introduced into the thoracic cavity through a 5-mm laparoscopic trocar outside of a plastic wound protector and the assistant stood at a foot-stool. The usage of trocar made it labor saving for the assistant when holding the thoracoscope, which also wouldn’t be blurred vulnerably when introduced into or out of the thoracic cavity. Moreover, when the thoracoscope assistant stood at a foot-stool, he was staggered with the surgeon and he never needed to step aside ceaselessly or stand in a distorted posture to make room for the surgeon.
Operative aspects and postoperative management
Thoracic exploration was performed before approaching to the target tissues. The technique of suction conducted dissection via energy instrumentation was applied to reduce bothersome bleeding and to maintain a clean field. We stuck to the principle of single direction when performing anatomical lung resection (4). We also developed a novel method of intrathoracic vertical overhanging approach to make the placement of endo-stapler easier when handling bronchus or pulmonary vessels (5). The method of “non-grasping en bloc mediastinal lymphadenectomy” was applied to simplify the instrumentation and make mediastinal lymphadenectomy more comfortable (4).
After the operation, one chest tube was placed at the posterior part of the incision for drainage. Extrapleural intercostal nerve block anesthesia to the incision using 7.5 mg/mL ropivacaine was administered during the wound closure. Most patients were extubated at the end of surgery and transferred to the ward while those with severe comorbidities would be transferred to intensive care unit (ICU) for further monitoring. All patients were provided with multidisciplinary analgesia including patient-controlled analgesia (PCA), transdermal and/or oral analgesics, postoperatively. The drainage tube was removed when there was less than 300 mL fluid every 24 hours and no air leakage was identified. Each patient was discharged with no main complications.
Comment
SP-VATS procedure was technically demanding during early days of practice. Accompanied difficulties were limited accesses, different vision, different operative method, and coordination between the thoracoscope assistant and the surgeon. The first encountered problems were the body position and placement of the incision. Lin et al. (6) placed the patient in a semi-prone position to lower and relax both of his and the thoracoscope assistant’s hands. This method made it labor saving for both of the surgeon and the assistant. However, it’s involved with totally different operative strategies that most surgeons were not quite familiar with. Most surgeons chose to place the patient in a lateral decubitus position the same as that during multi-portal VATS procedure (7). Since there was only one port available, the placement of the incision should take both the working distance and angle into the consideration. To provide facilities for procedures around the hilum, upper and lower mediastinal areas, as most surgeons did we also chose to place the incision in the 4th or 5th intercostal space between the anterior and middle axillary lines, where the intercostal space was wide enough for instrumentation. During the early stages we used a retractor to minimize iatrogenic touch during the introduction of instruments to the surgical field, especially in the cases of patients with thick chest wall. Then we found that a soft plastic wound protector would be useful to immobilize the thoracoscope and prevent potentially iatrogenic contamination of malignant cells to the incision.
To get a consistent vision with the surgeon, the assistant should stand at the same side with the surgeon. Usually the assistant should change position of the thoracoscope accordingly to facilitate the surgeon’s operation (8). Changing the position of the thoracoscope accordingly could sometimes help facilitate finding visualization and dissection of structures, as well as finding the correct angles for endo-stapler placement. However, the thoracoscope might be vulnerable to be blurred during this process. With the strategy of single-direction dissection and the method of intrathoracic vertical overhanging approach, we hardly needed to change the position of the thoracoscope to find appropriate visualization. In addition, it’s really crowded when both of the surgeon and assistant stood in such a limited space. The assistant usually needed to step aside holding the thoracoscope by lifting his hands, and this gesture made it uncomfortable to adjust the thoracoscope when needed. To solve these problems, a lengthened and thin thoracoscope, a trocar, and a foot-stool were helpful. The thin thoracoscope would save more space for other instruments. Indeed, a greater extracorporeal length (lengthened scope) necessitates more angular movement and higher angular velocity at the elbow. The longer the thoracoscope was the further the assistant could stand away from the surgeon. Moreover, when the thoracoscope assistant stood at a foot-stool, he was staggered with the surgeon and he never needed to step aside ceaselessly or adopt a distorted posture to make room for the surgeon. The usage of trocar made it labor saving for the assistant when holding the thoracoscope, which also wouldn’t be blurred vulnerably when introduced into or out of the thoracic cavity.
There were several limitations of this study. This was not a prospective study, so that we didn’t summarize the exact number of experience at each stage. Moreover, we felt regret that there were no specific data to prove the advantages of the strategy at stage IV. It’s hard to describe the feeling of labor saving and adopting a comfortable position by quantitative data.
In short, with gradual evolution of techniques during practice we found it more comfortable when performing SP-VATS procedure. Of course, the strategies for placement and holding of the thoracoscope might be different among different centers. Our experience might still be helpful for facilitating a beginning in practice of SP-VATS procedure for those beginners.
Acknowledgements
Funding: This paper was supported by the Key Science and Technology Program of Sichuan Province, People’s Republic of China (No. 2013SZ0005 and No. 2014SZ0148, both to Dr. Lunxu Liu).
Footnote
Conflicts of Interest: This paper was presented (oral presentation) at the 24th Annual Meeting of Asian Society for Cardiovascular and Thoracic Surgery (ASCVTS), Taipei, Apr 4th–10th, 2016.
References
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Gonzalez D, Delgado M, Paradela M, et al. Uni-incisional video-assisted thoracoscopic left lower lobectomy in a patient with an incomplete fissure. Innovations (Phila) 2011;6:45-7. [Crossref] [PubMed]
- Tu CC, Hsu PK. Global development and current evidence of uniportal thoracoscopic surgery. J Thorac Dis 2016;8:S308-18. [PubMed]
- Liu C, Ma L, Lin F, et al. Single-staged uniportal VATS major pulmonary resection for bilateral synchronous multiple primary lung cancers. J Thorac Dis 2014;6:1315-8. [PubMed]
- Guo C, Liu C, Lin F, et al. Intrathoracic vertical overhanging approach for placement of an endo-stapler during single-port video-assisted thoracoscopic lobectomy†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i84-6. [PubMed]
- Lin Z, Xi J, Xu S, et al. Uniportal video-assisted thoracic surgery lobectomy in semiprone position: primary experience of 105 cases. J Thorac Dis 2015;7:2389-95. [PubMed]
- Ng CS, Rocco G, Wong RH, et al. Uniportal and single-incision video-assisted thoracic surgery: the state of the art. Interact Cardiovasc Thorac Surg 2014;19:661-6. [Crossref] [PubMed]
- Ng CS, Wong RH, Lau RW, et al. Minimizing chest wall trauma in single-port video-assisted thoracic surgery. J Thorac Cardiovasc Surg 2014;147:1095-6. [Crossref] [PubMed]


