Endobronchial ultrasonography with guide sheath versus computed tomography guided transthoracic needle biopsy for peripheral pulmonary lesions: a propensity score matched analysis
Introduction
Lung cancer is the leading cause of cancer-related death worldwide (1). With the wide use of computed tomography (CT), more and more peripheral pulmonary lesions (PPLs) are found during population-based screening program (2). It is necessary to get a histological diagnosis of newly emerged PPLs in order to treat the patient appropriately. Presently, CT-guided transthoracic needle aspiration (CT-TTNA) is widely used in the histological diagnosis of PPLs and its sensitivity for diagnosing lung cancer remains as high as 90% (3). Meanwhile, the relatively high rate of complications such as pneumothorax, hemoptysis, pulmonary hemorrhage and poor tolerance in patients should not be overlooked (3,4).
In 2004, Kurimoto et al. (5) first reported the use of endobronchial ultrasonography with guide sheath (EBUS-GS) in diagnosing PPLs, and the sensitivity of EBUS-GS was 81%. The following studies reported that the diagnostic yield of EBUS-GS for PPLs was between 57% and 90%, and it was proved to be a safe procedure with low rate of pneumothorax and hemoptysis (6-21). Till now there is only one randomized controlled study (22) comparing the two procedures. However, the study did not complete the enrollment of patients on schedule. It is controversial which is a better choice for diagnosing PPLs without enough evidence. Hence we conducted a retrospective analysis of a prospective registry with propensity score matching to compare the complications and diagnostic accuracy of EBUS-GS and CT-TTNA for diagnosing PPLs. In this study, we hypothesized that EBUS-GS was not inferior to CT-TTNA in diagnosing of PPLs, but the complication rate of EBUS-GS was lower than CT-TTNA.
Methods
Study design
This prospective registry study was approved by Ethics Committee of Peking University People’s Hospital. Patients with PPLs were consecutively divided into EBUS-GS group and CT-TTNA group according to patients’ intent to treatment. All patients were given informed consent. The primary endpoint was complication rates of EBUS-GS and CT-TTNA. Then the diagnostic yields and sensitivity were second endpoints.
The inclusion criteria of the study were as follows: (I) PPLs presented as nodule or mass in the outer 1/3 pulmonary field on CT imaging, invisible under routine bronchoscope; (II) no pathological results were obtained before biopsy procedure. The exclusion criteria were: (I) presence of contraindications for EBUS-GS or CT-TTNA; (II) lesions presented as exudative or consolidation in CT imaging; (III) presence of complications such as pneumothorax or pleural effusion.
The diagnostic results were recorded as positive when they were malignant or consistent with the following operational histological results. If the patient did not undergo surgical treatment, the results would be decided by the following diagnostic treatment or imaging follow-up. The diagnostic value was determined by sensitivity, specificity, and accordance rate.
Equipment and procedure technique
The equipment for EBUS-GS included bronchoscope BF-P260F (Olympus, Tokyo, Japan), endobronchial ultrasonic system EU-M30S (Olympus, Tokyo, Japan), ultrasonic probe UM-S20-17S (Olympus, Tokyo, Japan), and guide sheath kit K201 (Olympus, Tokyo, Japan). The EBUS-GS biopsy procedure was performed by an experienced thoracic surgeon. The patient was in supine position, under general anesthesia, and provided ventilation through laryngeal mask. First, the operator placed bronchoscope into the bronchus of interest, inserted the probe covered with guided sheath through a work channel. Later, adjusted the bronchoscope and probe to obtain classical ultrasonic graphs. The assistant fixed the bronchoscope, pulled the probe out, and placed the brush and biopsy forceps into the guide sheath for biopsy, respectively. This process was assisted by X-ray fluoroscope. The specimen was sent for cytology and histological pathology diagnosis.
In terms of CT-TTNA, the main devices of CT-TTNA included Lightspeed VCT 64 spiral CT (GE Co., US) and Quick Core Suit (COOK Co., US). The outer diameter of the needle was 18G and the needle was fired manually. The procedure of CT-TTNA in our study were as follows: (I) The patient was located in supine, lateral or prone position depended on the location of lesion, and lidocaine was used for local anesthesia; (II) the patient received the first CT scan to mark the lesion on the skin and the surgeon measured the distance from chest wall to the lesion; (III) the surgeon inserted the needle according to the preset angle and depth, then the patient underwent the second CT scan and the operator modified the angle of the needle; (IV) firing the needle gun and repeating biopsy. The specimen was sent for histological pathology diagnosis.
Statistical analysis
Patients’ characteristics and perioperative data were compared by using Student’s t-test, Pearson χ2 test, and Fisher’s exact test. In order to eliminate the intergroup bias, age, gender, tumor size, depth of lesions from the chest wall, and pulmonary comorbidity were used during propensity scores matching (1:1). Two-tailed P values of 0.05 were considered as statistically significant. SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) was used to perform statistical analyses.
Results
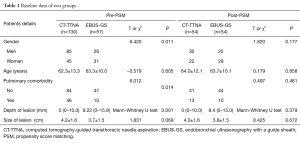
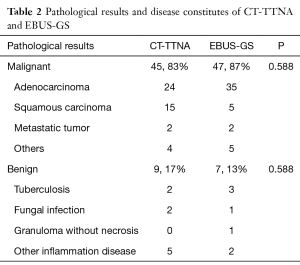
From June 2014 to December 2015, 187 consecutive patients were prospectively registered in this study, of which 57 patients underwent EBUS-GS and 130 patients underwent CT-TTNA. We conducted a retrospective analysis with propensity score matching of age, gender, preoperative pulmonary comorbidity, tumor size, and depth of lesions from the chest wall. 54 paired patients were obtained in each group. The baseline data were comparable between the two groups after matched (Table 1). More than 70% patients were diagnosed as adenocarcinoma and squamous carcinoma in two groups. Benign diseases were 17% (9/54) in CT-TTNA and 13% (7/54) in EBUS-GS respectively (Table 2).
Full table
Full table
The overall diagnostic yield was 81% (44/54) for EBUS-GS and 87% (47/54) for CT-TTNA. The difference was not statistically significant (χ2=0.628, P=0.428). The sensitivity for malignancy was 79% (37/47) for EBUS-GS and 93% (42/45) for CT-TTNA (P=0.04). Similarly, the false negative rate of CT-TTNA were significantly lower than the EBUS-GS (CT-TTNA: 7% vs. EBUS-GS: 21%, P=0.04). The specificity and positive predictive value was 100% in both groups.
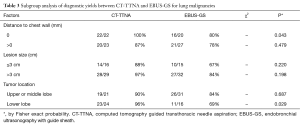
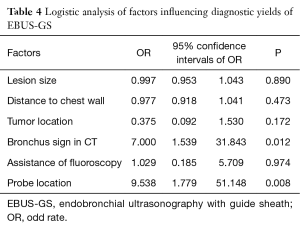
In the subgroup analysis of diagnostic yield between the two groups, the diagnostic sensitivity of lesions close to the chest wall was significantly higher in CT-TTNA than in EBUS-GS (100% vs. 80%, P=0.04), similar result was seen when tumor located in lower lobe (Table 3). Factors, which would probably influence the diagnostic accuracy of EBUS-GS, such as lesion size, distance of lesion to chest wall, location, bronchus sign in CT scan, assistance of fluoroscopy, and probe location, were analyzed in logistic regression. The result revealed that bronchus sign in CT scan and probe located in the tumor could increase the diagnostic accuracy of EBUS-GS (Table 4).
Full table
Full table
Considering the complications, only one case of hemoptysis after EBUS-GS was observed, and the patient was cured by hemocoagulase. No other complications occurred in EBUS-GS group. Hence, the complication rate was 2% (1/54) for EBUS-GS. Meanwhile, the complication rate of CT-TTNA was 13% (7/54), which included 5 cases of pneumothorax and 2 cases of hemoptysis. Two of the five pneumothorax patients received thoracic closed drainage and another three were treated by oxygen therapy and close monitoring. Two cases of hemoptysis received hemocoagulase. However the complication rate of CT-TTNA was not significantly higher than that of EBUS-GS (P=0.06).
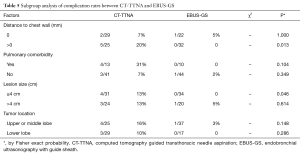
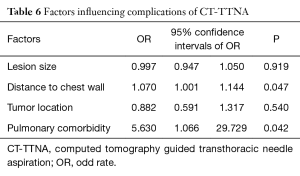
Subgroup analysis of complication rates showed that when the lesions were ≤4 cm or apart from the chest wall, the complication rate for CT-TTNA was significantly higher than EBUS-GS (Table 5). However, there were no significant differences when comparing complication rates of the two groups with pulmonary comorbidity and different tumor locations. In the CT-TTNA group, multivariate logistic analysis showed that the lesion close to the chest wall and pulmonary comorbidity were risk factors of occurring complications (Table 6).
Full table
Full table
Discussion
An ideal method comparing EBUS-GS and CT-TTNA should be a self-control study. But such designed study could hardly meet the requirement of ethical board and is difficult to carry out. In 2012, a randomized controlled study (22) reported that the sensitivity of EBUS-GS was comparable to CT-TTNA in diagnosing lung malignant nodules, but it did not summon enough patients on schedule to draw a more persuasive conclusion. In our prospective registered study, patients with PPLs were divided into EBUS-GS group and CT-TTNA group according to patients’ intent to treatment, and then we conducted a retrospective analysis with PSM. Patients of the two groups were matched by propensity score. The main reason for using retrospective analysis with PSM instead of a randomized designed study was to simplify the research process and speed up patients enrollment.
After PSM, patients’ age, tumor size, depth from chest wall, and pulmonary comorbidity were comparable between two groups. For all diseases, the diagnostic yield of EBUS-GS was comparable to CT-TTNA statistically. However, the diagnostic sensitivity was higher for CT-TTNA in malignancy. Subgroup analysis showed that CT-TTNA had a relatively higher sensitivity in diagnosis of PPLs close to chest wall or located in lower lobe. The size of the lesion did not significantly affected the diagnostic accuracy between two groups. The above results indicated that both of the two procedures were effective in the diagnosis of peripheral pulmonary lesions, but for malignant tumors, CT-TTNA had higher sensitivity. Reasons accounting for this result were probably as follows. Firstly, CT had a more precise positioning accuracy than ultrasound, meanwhile EBUS-GS biopsy cannot be monitored in real time. On the other hand, it was hardly for forceps to reach inside the tumors and the tissue volume grasped by forceps may be not enough to get an effective pathological diagnosis. On the contrary, the needle was easy to reach the core of a lesion and could get more tissue for diagnosis. Given the above deficiencies, the sensitivity of EBUS-GS for lung cancer was 79%, which was a relatively satisfactory diagnostic process for PPLs. In addition, if the lesion had a bronchus sign on CT scan, the diagnostic sensitivity could be as high as 94%, which was similar to CT-TTNA. Many previous studies (5,7,14-16) also suggested that bronchus sign or the probe located in the center of the lesion were positive factors for a higher diagnostic yields of EBUS-GS. Besides, the application of virtual bronchoscope and electromagnetic navigation bronchoscope could assist the probe to reach the target lesion and improve diagnostic accuracy (23-25).
The complication rate of EBUS-GS was 2%, which was not significantly lower than CT-TTNA (13%) in this study. However, we can see the difference directly. It should be the relatively small of sample size why the complication rates were not significantly different. Further, according to our clinical observation, the severity of complications between the two groups was not the same. In EBUS-GS group, the patient only had mild symptoms and the prognosis was fine. On the contrary, patients undergone CT-TTNA had relatively heavy complications which needed more complex treatment and more closely monitoring. In 2015, a retrospective study (10) analyzed complications caused by EBUS-GS in 965 patients. The total complication rate was 1.3%, pneumothorax was 0.8% and pneumonia was 0.5%. No arrhythmia, hypotension, hypoxemia occurred during EBUS-GS procedures. A randomized trail from Fielding D.I. (22) also proved that EBUS-GS was a relatively safe procedure.
Subgroup analysis of complications revealed that when the lesion was apart from the chest wall, the complication rate of CT-TTNA was significantly higher than EBUS-GS. Meanwhile, intergroup analysis of CT-TTNA group showed that pulmonary comorbidity and deep lesions were associated with high complications of CT-TTNA. Heyer (26) reported that a small and deep nodule meant a relatively high risk of pneumothorax, and the existence of emphysema was a predictor for pulmonary hemorrhage. Laurent F (27) confirmed that a deeper depth was independent risk factor for pneumothorax. These conclusions were consistent with this study. Therefore, it was recommended that CT-TTNA should be avoided if the lesion was not close to the chest wall and if a patient has pulmonary comorbidity, such as emphysema and interstitial lung disease.
The present study has some limitations. First, it was hard to completely eliminate the selection bias caused by the grouping of patients, in spite of the usage of PSM. Second, more patients are needed to fulfill the subgroup analysis divided by PPLs’ size.
Conclusions
Both EBUS-GS and CT-TTNA are effective in diagnosis of PPLs, but the sensitivity for malignancy is higher in CT-TTNA. EBUS-GS has fewer complications, which is suitable for patients combined with pulmonary diseases and lesions with bronchus signs. CT-TTNA is appropriate for lesions close to chest wall.
Acknowledgements
Funding: This study was supported by the Capital Health Development and Scientific Research Project from Beijing Health and Family Planning Commission [2014-2-4084].
Footnote
Conflicts of Interest: This study was presented at the 24th European Conference on General Thoracic Surgery, Naples, Italy, May 29th~June 1st, 2016.
Ethics statement: This prospective registry study was approved by Ethics Committee of Peking University People’s Hospital (No. 2015PHB028-01).
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Hiraki T, Mimura H, Gobara H, et al. CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions performed with 20-gauge coaxial cutting needles: diagnostic yield and risk factors for diagnostic failure. Chest 2009;136:1612-7. [Crossref] [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6 Suppl 1:S99-S107. [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [Crossref] [PubMed]
- Kikuchi E, Yamazaki K, Sukoh N, et al. Endobronchial ultrasonography with guide-sheath for peripheral pulmonary lesions. Eur Respir J 2004;24:533-7. [Crossref] [PubMed]
- Shirakawa T, Imamura F, Hamamoto J, et al. Usefulness of endobronchial ultrasonography for transbronchial lung biopsies of peripheral lung lesions. Respiration 2004;71:260-8. [Crossref] [PubMed]
- Paone G, Nicastri E, Lucantoni G, et al. Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions. Chest 2005;128:3551-7. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Becker HD, et al. Endobronchial ultrasound-guided transbronchial lung biopsy in fluoroscopically invisible solitary pulmonary nodules: a prospective trial. Chest 2006;129:147-50. [Crossref] [PubMed]
- Yamada N, Yamazaki K, Kurimoto N, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest 2007;132:603-8. [Crossref] [PubMed]
- Yoshikawa M, Sukoh N, Yamazaki K, et al. Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X-ray fluoroscopy. Chest 2007;131:1788-93. [Crossref] [PubMed]
- Chung YH, Lie CH, Chao TY, et al. Endobronchial ultrasonography with distance for peripheral pulmonary lesions. Respir Med 2007;101:738-45. [Crossref] [PubMed]
- Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902-10. [Crossref] [PubMed]
- Shinagawa N, Nakano K, Asahina H, et al. Endobronchial ultrasonography with a guide sheath in the diagnosis of benign peripheral diseases. Ann Thorac Surg 2012;93:951-7. [Crossref] [PubMed]
- Izumo T, Sasada S, Chavez C, et al. The diagnostic utility of endobronchial ultrasonography with a guide sheath and tomosynthesis images for ground glass opacity pulmonary lesions. J Thorac Dis 2013;5:745-50. [PubMed]
- Li M, Peng A, Zhang G, et al. Endobronchial ultrasound transbronchial lung biopsy with guide-sheath for the diagnosis of peripheral pulmonary lesions. Zhonghua Jie He He Hu Xi Za Zhi 2014;37:36-40. [PubMed]
- Ikezawa Y, Sukoh N, Shinagawa N, et al. Endobronchial ultrasonography with a guide sheath for pure or mixed ground-glass opacity lesions. Respiration 2014;88:137-43. [Crossref] [PubMed]
- Takai M, Izumo T, Chavez C, et al. Transbronchial needle aspiration through a guide sheath with endobronchial ultrasonography (GS-TBNA) for peripheral pulmonary lesions. Ann Thorac Cardiovasc Surg 2014;20:19-25. [Crossref] [PubMed]
- Hayama M, Izumo T, Matsumoto Y, et al. Complications with Endobronchial Ultrasound with a Guide Sheath for the Diagnosis of Peripheral Pulmonary Lesions. Respiration 2015;90:129-35. [Crossref] [PubMed]
- Izumo T, Sasada S, Chavez C, et al. The diagnostic value of histology and cytology samples during endobronchial ultrasound with a guide sheath. Jpn J Clin Oncol 2015;45:362-6. [Crossref] [PubMed]
- Chavez C, Sasada S, Izumo T, et al. Endobronchial ultrasound with a guide sheath for small malignant pulmonary nodules: a retrospective comparison between central and peripheral locations. J Thorac Dis 2015;7:596-602. [PubMed]
- Fielding DI, Chia C, Nguyen P, et al. Prospective randomised trial of endobronchial ultrasound-guide sheath versus computed tomography-guided percutaneous core biopsies for peripheral lung lesions. Intern Med J 2012;42:894-900. [Crossref] [PubMed]
- Asano F, Matsuno Y, Tsuzuku A, et al. Diagnosis of peripheral pulmonary lesions using a bronchoscope insertion guidance system combined with endobronchial ultrasonography with a guide sheath. Lung Cancer 2008;60:366-73. [Crossref] [PubMed]
- Tamiya M, Okamoto N, Sasada S, et al. Diagnostic yield of combined bronchoscopy and endobronchial ultrasonography, under LungPoint guidance for small peripheral pulmonary lesions. Respirology 2013;18:834-9. [Crossref] [PubMed]
- Asano F, Shinagawa N, Ishida T, et al. Virtual bronchoscopic navigation improves the diagnostic yield of radial-endobronchial ultrasound for peripheral pulmonary lesions with involved bronchi on CT. Intern Med 2015;54:1021-5. [Crossref] [PubMed]
- Heyer CM, Reichelt S, Peters SA, et al. Computed tomography-navigated transthoracic core biopsy of pulmonary lesions: which factors affect diagnostic yield and complication rates? Acad Radiol 2008;15:1017-26. [Crossref] [PubMed]
- Laurent F, Michel P, Latrabe V, et al. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: incidence and risk factors. AJR Am J Roentgenol 1999;172:1049-53. [Crossref] [PubMed]


