Classification of drugs to treat multidrug-resistant tuberculosis (MDR-TB): evidence and perspectives
Multidrug-resistant (MDR) tuberculosis (TB) (defined as resistance to at least isoniazid and rifampicin), has a relevant epidemiological impact, with 480, 000 cases and 190,000 deaths notified in 2014; 10% of them meet the criteria for extensively drug-resistant (XDR)-TB [MDR-TB with additional resistance to any fluoroquinolone, and to at least one injectable second-line drugs (SLDs)] (capreomycin, kanamycin or amikacin) (1,2).
The fight against MDR-TB is one of the eight core interventions to target TB elimination (3,4). In spite of the progresses achieved, no more than 60% among MDR-TB cases, 40% among XDR-TB cases and <20% among cases with resistance patterns beyond XDR-TB achieve treatment success (5,6). Furthermore, in its 2015 report (1), WHO underlined as out of 480,000 MDR-TB cases, only 25% have been detected [123,000] and 50% cured.
Anti-MDR/XDR-TB regimens are still very long, toxic and expensive, although recently shorter regimens have been recommended (7,8).
Previous World Health Organization (WHO) guidelines were classifying anti-TB drugs into five main groups, based on a hierarchy of safety and/or the effectiveness considerations (2,7,9). This classification originated in 2006, and updated in 2008, 2011 and, finally, in 2016 based on new evidence, particularly from the former group 5 drugs (10-31).
Rationale basis of anti-TB treatment
The historical principles, derived from randomized clinical trials (RCTs), are still valid: (I) combining different effective drugs to prevent the selection of resistant mutants of M. tuberculosis; and (II) prolonging the treatment to sterilise the infected tissues and, therefore, prevent relapse (1,9,10).
At least four drugs likely to be effective compose the regimen, of which at least two are essential (or ‘core’ drugs), while two are companion drugs (2,10,11). The core drugs are those with the capacity to kill M. tuberculosis in any of its metabolic phases. In contrast, the role of the companion drugs is to support the core ones, protecting their action and avoiding selection of further resistance. Whilst one of the core drugs should have a good bactericidal activity, the other should have a good sterilising activity, and they need to be maintained for the entire duration of treatment. While bactericidal drugs efficiently reduce the bulk of the rapidly multiplying bacilli (decreasing infectiousness and avoiding the disease’s progression), sterilising drugs take care of the population of dormant and semi-dormant bacilli, allowing cure and preventing relapse. The best sterilising drugs may reduce the duration of the treatment while the companion drugs are no longer necessary after bacteriological conversion (2,10,11). When documented resistance or toxicity appears for a core drug, it should be replaced by another with a similar efficacy (bactericidal and sterilising). Similarly, an accompanying drug should be replaced by another with a similar action.
Classification of anti-TB drugs and history of its update
The choice of drugs is based on their efficacy and toxicity. Based on this principle, the 2008 and then the 2011 WHO guidelines proposed a range of five groups, from group 1 (which included first-line drugs) to groups 2–5 which included SLDs. Group 5 included the drugs with potentially limited clinical efficacy or limited evidence (2,5,10,11) (Figure 1). In order to show clearly the present and future evolution of the drugs grouping, we have arbitrarily included in the Figure 1, under group 5 drugs, the new drugs delamanid and bedaquiline, for which, till very recently, limited evidence was available.
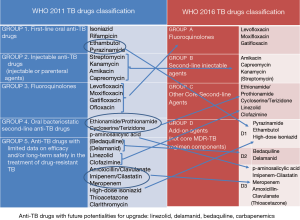
In a recent publication (2), a possible new classification was proposed by Caminero and Scardigli (Table 1) while the new 2016 WHO classification proposed a hierarchy of five groups (7), including groups A to D: fluoroquinolones are in group A, second-line injectables in group B, other core SLDs in group C and add-on agents in group D (where drugs are divided into the subgroups D1, D2 and D3) (Figure 1). This classification is specifically designed for rifampicin-resistant (diagnosed via Xpert) or MDR-TB cases (7).
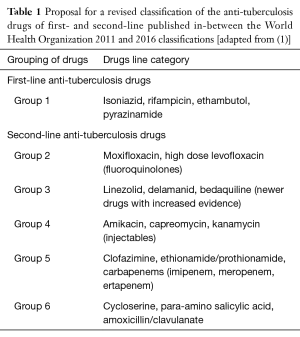
Full table
This manuscript, belonging to the ERS/ALAT SinTB project (a research coordination project), describes the rationale for the shift from the 2008 and 2011 to the 2016 WHO classifications (Figure 1), while discussing possible future evolutions.
Group 1 versus group A
All potentially effective ex-group 1 drugs need to be included in the regimen, considering that isoniazid, rifampicin and pyrazinamide are core drugs, and ethambutol is a companion drug. High-dose isoniazid should be added to an MDR/XDR-TB regimen when the katG mutation is not documented by the genotype line probe assay (Quest Diagnostics, Madison, NJ, USA) but it should not be counted among the four active drugs (2,10,11).
However, very recent evidence suggests this might not be fully true, which might further support adding systematically high dose isoniazid, even in the presence of katG mutation (12).
InhA mutation, in fact is conferring a very low level of resistance (which is reasonably compatible with normal doses of isoniazid), while katG mutation is responsible of varying levels of drug resistance, most of these strains having low or intermediate level of resistance. Therefore, in quite many cases of isoniazid resistance in the presence of katG mutation, high dose isoniazid is likely to be effective (12).
Pyrazinamide should always be used, although its drug susceptibility test is unreliable, not being counted as well among the four active drugs (2,10,11).
In the new classification, and for the purpose of treating MDR-TB, pyrazinamide and ethambutol have been included in group D1 (7) (Figure 1).
Fluoroquinolones (particularly the later-generation ones: high-dose levofloxacin or moxifloxacin) are core drugs, having bactericidal and sterilising activity. They ensure good tolerability and predict a favourable outcome in MDR-TB treatment (2,10,11,13).
Based on these features they have been promoted in group A (7).
Group 2 versus group B
The ex-group 2 included the injectable SLDs (2,10,11), having bactericidal (but not sterilising) activity and a safety profile worse than fluoroquinolones (cumulative toxicity leading to deafness or kidney problems). They have been included in group B, which immediately follows fluoroquinolones (2) (Figure 1). The possibility to use streptomycin in exceptional cases to treat MDR-TB (especially in selected XDR-TB cases not using previously this drug and with drug susceptibility testing showing susceptibility) might deserve further discussion.
Group 3 and 4 versus group C
As ex-group 1 drugs were not symmetrically considered for MDR-TB treatment, the following group in hierarchical order was group 4, which included ethionamide/prothionamide, cycloserine/terizidone and PAS.
Having bactericidal activity, they are core SLDs although with high incidence of adverse events, some of them very severe. For this reason, they have been included in group C, except PAS, which has been downgraded to group D3 because of the worse tolerability in this group of drugs (7).
Due to the improved evidence on their efficacy and tolerability, linezolid and clofazimine have been upgraded to group C (7).
Because of the new recent evidence, a special discussion is necessary for linezolid, delamanid and bedaquiline.
Linezolid
Linezolid is a core oral drug. Increasing evidence on its efficacy is accumulating, including meta-analyses and two RCTs, in addition to observational studies. Unfortunately, the current cost and the documented toxicity (14-22) have been a barrier to its wider use. However, the cost of a generic, quality-assured compound is decreasing (21) and a recent report suggests that tolerability can be increased lowering the initial dose or adjusting it during treatment [e.g., using therapeutic drug monitoring (TDM)] (23).
Delamanid and bedaquiline
Information on a favourable safety and efficacy profile are accumulating for both delamanid and bedaquiline, including individual use as per existing recommendations (24), use beyond 6 months (24) and in children (25), and even combined use (26).
Bedaquiline is a core drug, targeting both actively replicating and dormant bacilli. The available evidence includes RCTs (27,28) and observational studies, including experiences from compassionate use programmes as well (26,29). Bedaquiline accelerates bacteriological conversion while increasing the proportion of converters and the cure rates (27,28). The main concern regarding safety of bedaquiline is the unexplained higher number of deaths in the bedaquiline arm of the RCT (28), as its commonest adverse reaction is the QTc interval increase in the electrocardiogram (ECG) (24,27,28). Important to mention, its cross-resistance with clofazimine, although recent data seem to indicate it might be non-clinically relevant (22).
Delamanid because of its bactericidal and sterilising activity, can also be considered a core drug (30,31). It does not show cross-resistance with other anti-TB drugs (30,31), is effective in increasing both bacteriological conversion and treatment outcomes, and does reduce mortality (30-32). As it also increases QTc, ECG monitoring is necessary likewise bedaquiline.
Recent anecdotal evidence has been provided that both drugs can be given for more than 6 months, that delamanid is safe in children and that the two drugs might be combined (25,26,33).
Which drugs might be upgraded
Linezolid, bedaquiline and delamanid might be able to change the bleak prognosis of MDR-TB patients with resistance to fluoroquinolones (some clinicians call these cases pre-XDR, using a non-approved definition) (2) (Figure 1).
Recently new evidence has been made available on carbapenems, with the profile of a companion drug. Imipenem/cilastatin and meropenem, combined with clavulanic acid, seem to have a promising activity, while being well tolerated (34-37). In the direct comparison meropenem performs better than imipenem (37). Initial clinical experience with ertapenem suggests that it can be a valid drug for the home-care phase of MDR-TB treatment, as it can be administered intramuscularly once a day (38).
Important to note that recent evidence suggests this category of drugs might have, in view of its bactericidal activity, the role of a core drug (39).
Future scenarios
Linezolid, delamanid and bedaquiline might acquire a more prominent role in MDR-TB treatment both in adults and in children, given their ‘core drug’ profile. Under specific conditions delamanid and bedaquiline might be considered for combined use (26), although further RCTs’ evidence is necessary.
Further studies are also necessary to establish if high dose moxifloxacin, and rifabutin, might play a future role in the MDR-TB armamentarium.
We hope that further evidence will be accompanied by decreasing costs of these compounds; the recent inclusion of both of them in the Global Drug Facility list of pre-qualified drugs is encouraging.
Acknowledgements
None.
Footnote
Conflicts of Interest: This manuscript is part of the activities of the ERS/ALAT SinTB project.
Disclaimer: The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions and policies of their institutions.
References
- World Health Organization. Global tuberculosis report. 2015. Available online: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf
- Caminero JA, Scardigli A. Classification of antituberculosis drugs: a new proposal based on the most recent evidence. Eur Respir J 2015;46:887-93. [Crossref] [PubMed]
- Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J 2015;45:928-52. [PubMed]
- D'Ambrosio L, Dara M, Tadolini M, et al. Tuberculosis elimination: theory and practice in Europe. Eur Respir J 2014;43:1410-20. [Crossref] [PubMed]
- Falzon D, Gandhi N, Migliori GB, et al. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J 2013;42:156-68. [Crossref] [PubMed]
- Migliori GB, Sotgiu G, Gandhi NR, et al. Drug resistance beyond extensively drug-resistant tuberculosis: individual patient data meta-analysis. Eur Respir J 2013;42:169-79. [Crossref] [PubMed]
- World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis. 2016 update. Available online: http://www.who.int/tb/MDRTBguidelines2016.pdf
- Sotgiu G, Tiberi S, D'Ambrosio L, et al. Faster for less: the new "shorter" regimen for multidrug-resistant tuberculosis. Eur Respir J 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Falzon D, Jaramillo E, Schünemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 2011;38:516-28. [Crossref] [PubMed]
- Caminero JA, Sotgiu G, Zumla A, et al. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis 2010;10:621-9. [Crossref] [PubMed]
- Caminero JA. editor. Guidelines for Clinical and Operational Management of Drug-Resistant Tuberculosis. Paris, France: International Union Against Tuberculosis and Lung Disease, 2013.
- Cambau E, Viveiros M, Machado D, et al. Revisiting susceptibility testing in MDR-TB by a standardized quantitative phenotypic assessment in a European multicentre study. J Antimicrob Chemother 2015;70:686-96. [Crossref] [PubMed]
- Johnston JC, Shahidi NC, Sadatsafavi M, et al. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One 2009;4:e6914. [Crossref] [PubMed]
- Sotgiu G, Centis R, D'Ambrosio L, et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J 2012;40:1430-42. [Crossref] [PubMed]
- Chang KC, Yew WW, Tam CM, et al. WHO group 5 drugs and difficult multidrug-resistant tuberculosis: a systematic review with cohort analysis and meta-analysis. Antimicrob Agents Chemother 2013;57:4097-104. [Crossref] [PubMed]
- Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 2012;367:1508-18. [Crossref] [PubMed]
- Tang S, Yao L, Hao X, et al. Efficacy, safety and tolerability of linezolid for the treatment of XDR-TB: a study in China. Eur Respir J 2015;45:161-70. [Crossref] [PubMed]
- Villar M, Sotgiu G, D'Ambrosio L, et al. Linezolid safety, tolerability and efficacy to treat multidrug- and extensively drug-resistant tuberculosis. Eur Respir J 2011;38:730-3. [Crossref] [PubMed]
- De Lorenzo S, Centis R, D'Ambrosio L, et al. On linezolid efficacy and tolerability. Eur Respir J 2012;39:770-2. [Crossref] [PubMed]
- Sotgiu G, Pontali E, Migliori GB. Linezolid to treat MDR-/XDR-tuberculosis: available evidence and future scenarios. Eur Respir J 2015;45:25-9. [Crossref] [PubMed]
- Sotgiu G, Centis R, D'Ambrosio L, et al. Low minimal inhibitory concentrations of linezolid against multidrug-resistant tuberculosis strains. Eur Respir J 2015;45:287-9. [Crossref] [PubMed]
- Cox H, Ford N. Linezolid for the treatment of complicated drug-resistant tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2012;16:447-54. [Crossref] [PubMed]
- Srivastava S, Peloquin CA, Sotgiu G, et al. Therapeutic drug management: is it the future of multidrug-resistant tuberculosis treatment? Eur Respir J 2013;42:1449-53. [Crossref] [PubMed]
- Pontali E, Sotgiu G, D'Ambrosio L, et al. Bedaquiline and multidrug-resistant tuberculosis: a systematic and critical analysis of the evidence. Eur Respir J 2016;47:394-402. [Crossref] [PubMed]
- Tadolini M, Garcia-Prats AJ, D'Ambrosio L, et al. Compassionate use of new drugs in children and adolescents with multidrug-resistant and extensively drug-resistant tuberculosis: early experiences and challenges. Eur Respir J 2016;48:938-43. [Crossref] [PubMed]
- Tadolini M, Lingtsang RD, Tiberi S, et al. First case of extensively drug-resistant tuberculosis treated with both delamanid and bedaquiline. Eur Respir J 2016;48:935-8. [Crossref] [PubMed]
- Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 2009;360:2397-405. [Crossref] [PubMed]
- Diacon AH, Pym A, Grobusch MP, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med 2014;371:723-32. [Crossref] [PubMed]
- Tiberi S, De Lorenzo S, Centis R, et al. Bedaquiline in MDR/XDR-TB cases: first experience on compassionate use. Eur Respir J 2014;43:289-92. [Crossref] [PubMed]
- Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 2012;366:2151-60. [Crossref] [PubMed]
- Skripconoka V, Danilovits M, Pehme L, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J 2013;41:1393-400. [Crossref] [PubMed]
- Wells CD, Gupta R, Hittel N, et al. Long-term mortality assessment of multidrug-resistant tuberculosis patients treated with delamanid. Eur Respir J 2015;45:1498-501. [Crossref] [PubMed]
- Esposito S, D'Ambrosio L, Tadolini M, et al. ERS/WHO Tuberculosis Consilium assistance with extensively drug-resistant tuberculosis management in a child: case study of compassionate delamanid use. Eur Respir J 2014;44:811-5. [Crossref] [PubMed]
- De Lorenzo S, Alffenaar JW, Sotgiu G, et al. Efficacy and safety of meropenem-clavulanate added to linezolid-containing regimens in the treatment of MDR-/XDR-TB. Eur Respir J 2013;41:1386-92. [Crossref] [PubMed]
- Tiberi S, Sotgiu G, D'Ambrosio L, et al. Effectiveness and Safety of Imipenem-Clavulanate Added to an Optimized Background Regimen (OBR) Versus OBR Control Regimens in the Treatment of Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis. Clin Infect Dis 2016;62:1188-90. [Crossref] [PubMed]
- Tiberi S, Payen MC, Sotgiu G, et al. Effectiveness and safety of meropenem/clavulanate-containing regimens in the treatment of MDR- and XDR-TB. Eur Respir J 2016;47:1235-43. [Crossref] [PubMed]
- Tiberi S, Sotgiu G, D'Ambrosio L, et al. Comparison of effectiveness and safety of imipenem/clavulanate- versus meropenem/clavulanate-containing regimens in the treatment of MDR- and XDR-TB. Eur Respir J 2016;47:1758-66. [Crossref] [PubMed]
- Tiberi S, D'Ambrosio L, De Lorenzo S, et al. Ertapenem in the treatment of multidrug-resistant tuberculosis: first clinical experience. Eur Respir J 2016;47:333-6. [Crossref] [PubMed]
- Diacon AH, van der Merwe L, Barnard M, et al. β-Lactams against Tuberculosis--New Trick for an Old Dog? N Engl J Med 2016;375:393-4. [Crossref] [PubMed]


