Primary adenosquamous carcinoma of the esophagus: an analysis of 39 cases
Introduction
Esophageal cancer is the 6th in male and 9th in female of estimated deaths among the cancers worldwide (1). More than 90% esophageal cancers are either squamous cell carcinoma or adenocarcinoma (2). The adenosquamous carcinoma (ASC) of the esophagus is an uncommon type of malignant esophageal neoplasm containing both squamous cell carcinoma (SCC) and adenocacinoma (AC) components, only seen in about 0.74% of all esophageal cancers with male predominated (3). The biological behavior, clinicopathological features and prognostic factors of ASC have been already discussed in several case reports or series (4-11). It seems that ASC has higher potential to regional lymph node metastasis, misdiagnosis and worse prognosis than SCC. However, the prognostic factor of ASC has remained unclear due to its extremely low incidence and small series reports (8-11). In this study, we collected 39 esophageal ASC cases with detailed clinical data from our hospital that underwent transthoracic esophagectomy to focus on its clinical characteristics and prognosis compared to SCC and AC.
Methods
Patients
A total of 3,855 patients with esophageal carcinoma underwent surgical resection between August 2005 and March 2014 in our department. Among them there were 39 patients (1.0%) with histologically confirmed primary ASC. The medical records of these 39 patients were analyzed retrospectively. This study was approved by the Ethics Committee of West China Hospital (No.201649).
Patients had a full work-up before operation, which mainly included physical examination, barium swallow, endoscopic biopsy, contrast computed tomography scan of chest and abdomen, etc. All patients underwent a radical intent esophagectomy, with three-field or two-field lymphadenectomy. For comparison, we used the propensity matched method (as a ratio of 1:1) to select 39 EAC patients and 39 ESCC patients among 3,855 patients underwent esophagectomy during the same period.
Histopathology
All resected specimens were pathologically examined by two senior pathologists. Tumor was classified as ASC based on the criteria of Japanese Society for Esophageal Disease (JSED). Both SCC component and AC component could be identified by light microscopy or immunohistochemistry, with each accounting for at least 20% of the area in the section including the deepest portion of tumor penetration (11). TNM stage was based on the 7th edition of the American Joint Committee on Cancer (AJCC) TNM staging system for ESCC (12). Tumor grade was defined as well differentiated, moderately differentiated, or poorly differentiated, according to the World Health Organization (WHO) classification of esophageal tumors (13)
Follow-up
After surgery, all patients were followed up every 3 months in the first year, every 6 months in the 2–3 years, and every year thereafter. Overall survival (OS) time was defined as the period from the date of surgery to the point of death or last contact.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). The chi-square or Fisher’s exact test was used to compare categorical data. A propensity score matched analysis was used to compensate for the differences in baseline characteristics between ASC, AC and SCC. Univariate analysis of survival was performed using the Kaplan-Meier method to estimate survival probabilities in patient subgroups. The log-rank test was used to assess differences in survival between groups. Cox proportional hazard regression model was used to perform a multivariate analysis. A two-side P value less than 0.05 was considered statistically significant.
Results
Patient characteristics
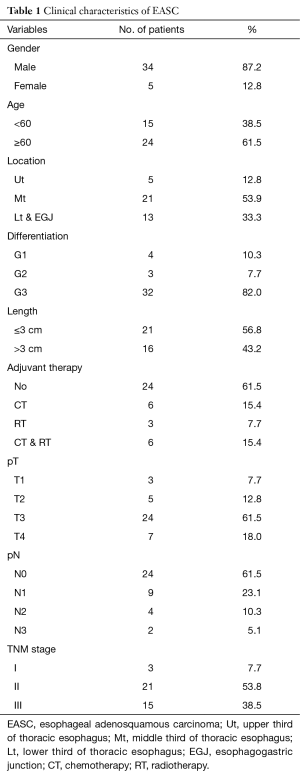
The clinicopathological features of 39 patients with primary ASC patients was shown in Table 1. It contained 34 men and 5 women with a median age of 61.0 years (range from 39~85). The average number of lymph nodes resected was 15.9. The clinical manifestations were similar to those of other types of esophageal cancer, with dysphagia, retrosternal or upper abdominal pain, and loss of body weight being the main presenting features. Of those 39 patients, 38 had endoscopic biopsy before surgery, and 35 (92.11%) were misdiagnosed as SCC [33] or others [2]. There was no operative death. Three patients were died within 3 months after operation. One patients received adjuvant radiochemotherapy with a tumor recurrence and died 46 days after operation. The other two patients were died 90 days after operation. The 90 days mortality was 7.7%. In our study, only one patients received neoadjuvant therapy. Mostly patients did not received neoadjuvant therapy due to high resectability evaluated preoperatively.
Full table
Among the 39 patients, 21 (53.9%) ASC located in middle thoracic esophagus, 13 (33.3%) located in lower or esophagogastric junction and 5 (12.8%) located in upper third. For tumor cell differentiation, 32 (82.1%) was poorly differentiated (G3), 3 (7.7%) moderately differentiated (G2), and 4 (10.6%) well differentiated (G1).
Based on the 7th edition of the AJCC TNM staging system for ESCC, 3 (7.7%) with lesion confined within mucosa (T1), 7 (18.0%) infiltrated muscularis propria (T2), 24 (61.5%) involved adventitia (T3), and 14 (10.3%) invaded adjacent structures (T4a). For pN stage, 24 (61.5%) patients had no lymph node metastasis (N0), 9 (23.1%) had 1-2 lymph nodes metastasis (N1), 4 (10.3%) had 3-6 positive nodes (N2), and 2 (5.1%) had more than 6 lymph nodes metastasis (N3). In addition, 3 (7.7%) cases were classified as stage I, 21 (53.9%) were as stage II, and 15 (38.5%) were as stage III with regarding to TNM staging.
For the post-operative treatment, 15 (38.5%) cases received adjuvant therapies (chemotherapy/radiotherapy/chemoradiotherapy: 6/3/6, respectively).
Survival analysis
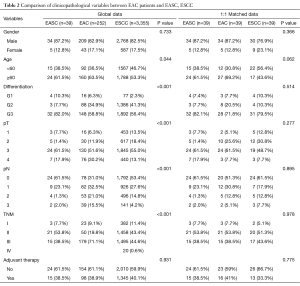
The overall median follow-up period was 30.0 months with 17 patients died and 3 cases were lost to follow-up. The median survival time (MST) was 44.4 months. The 1-, 3- and 5-year overall survival rates were 82.1%, 51.6% and 37.5%, respectively (Figure 1). Patients with esophageal AC and SCC were propensity matched at a ratio of 1:1 as control group according to sex, age, and TNM stage (Table 2). Compared to esophageal SCC and AC, there were no significant difference in survive time (P=0.616) (Figure 2).
Full table

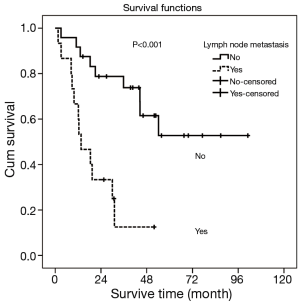
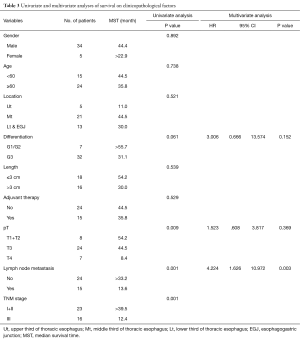
Univariate and multivariate analyses were performed to assess the relationship between clinicopathological features and the prognosis (Table 3). In univariate analysis, pT stage, lymph node metastasis and pTNM Stage showed the statistical difference. The MST for pT1+2 patients was 54.2 months while the MST for pT3 and pT4 stage were significant shorter (44.5 vs. 8.4 months, respectively, P=0.009). Patients with lymph node metastasis revealed a shorter survive time than patients without (MST: >33.8 vs. 13.6 months, respectively, P<0.001) (Figure 3). Also, patients with stage I and stage II has a better prognosis compared to stage III (MST: >39.5 vs. 12.4 months, respectively, P<0.001) (Figure 4). However, there were no significant differences in survival time with patients’ gender (P=0.892), age (P=0.738), tumor location (P=0.521), tumor cell differentiation (P=0.061), tumor length (P=0.539) and adjuvant therapy (P=0.529) in the univariate analyses.
Full table
Furthermore, multivariate analysis was conducted using the Cox proportional hazards mode, only lymph node metastasis (P=0.003; 95% CI: 1.626–10.972) was showed the significant differences (Table 3). Patients with lymph node metastasis indicated a shorter median survival time.
Discussion
ASC of the esophagus did not have an unique name in early period and was reported as adenoacanthoma firstly by Mcpeak et al. [1947] (14). Until to 1989, the 7th Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus (GCPSCE) attributed ASC to the “other malignancies” and were distinct from adenoacanthoma by JSED. In 8th GCPSCE [1992], JSED defined ASC as “adenosquamous carcinoma” and the mucoepidermoid carcinoma of the esophagus (MECE) which contained mucus secreting cells was classified to the subclass type of “adenosquamous carcinoma” (15). Until to the 9th GCPSCE [1999], MECE was distinguished from EASC. On the other side, the 1990 WHO classification of tumors of the esophagus defined the adenosuqamous carcinoma as where the adenocarcinomatous and squamous carcinomatous components were intermingled, and MECE was characterized by the presence of an intimate mixture of squamous cells and mucus secreting cells (16). Due to JESD and WHO defined the esophageal ASC respectively, there are two diagnostic criteria of ASC. The JESD criterion is that having at least 20% each of AC and SCC components under microscopic examination. The WHO criterion describes simply that ASC has a significant SC component that is intermingled with tubular AC elements, with no special reference to the ratio of these two components (13). The cases we collected were all adopted the JSED criterion, with the reason probably due to that JSED criterion defined an exact minimum proportion for both SCC and AC components.
The origin of esophageal ASC remains unclear. Some authors considered that esophageal ASC arises from esophageal gland cells or ductal cells. For the reason that epithelium and submucosal glands are all derived from the foregut during embryogenesis, the AC component has potential to metaplasia to SCC (17). Other authors considered that esophageal ASC arises from epithelium, where develop into SCC firstly and then glandular differentiate into ASC (4,18). Chen et al. (8) analyzed 37 esophageal ASC patients and found carcinoma differentiating in adjacent mucosa while no glandular differentiation in submucosal glands or ductal cells. Yachida et al. (11) found 10 of 18 ASC patients had intraepithelial SCC element contiguous to main lesion which suggested that ASC originates from squamous epithelium. It also has been speculated that ASC arisen from stem cells of basal layer of the squamous epithelium (19). Pera et al. (20) considered that chronic duodenal reflux may induce the development of metaplastic cell with glandular differentiation from the stem cells of squamous epithelium and also found ASC component in rat’s esophagus which underwent esophagojejunostomy 20 week ago.
ASC of the esophagus is a rare malignant carcinoma with a low proportion about 0.37–3% (5,8-11,21,22). In our study, ASC only took 1.01% (39/3,855) of all esophageal cancers in our database, which are consisted with those studies. The clinical manifestation and endoscope finding for esophageal ASC are similar to SCC and the diagnosis of ASC mainly depends on endoscopic biopsy. However, the misdiagnosis of ASC is frequently with a rate about 61.1–100% on reported studies (5,8-11). The reason is probably that SCC component of ASC mainly found in epithelium while AC component mainly occurred in submucosal gland or deeper portion of the tumor, where always could not get enough biopsy.
The 5-year OS rate of ASC is 39.0% in our study, which is similar to reported studies with the 5-year OS at 18.1–63.6%. Comparing with same period of the SCC and AC patients in our hospital, there were no significant difference in survive time (P=0.616) (Figure 2). Our result confirmed the findings of the previous work reported by Sun et al. (10). However, our results differ from some published studies, which found esophageal ASC has a worse prognosis than SCC and AC reported by Huang et al. (22) and Chen et al. (8). The reason may due to extensive nodal metastasis, lymphovascular and perineural invasion in Huang et al. (22). Compared to the similar analysis (37 ASC patients) reported by Chen et al. (8), the difference prognosis may due to different TNM stages and lower percent of patients who received adjuvant therapy. The sample size in all of those studies may also lead to these discrepancies. Therefore, further investigation for the prognosis of ASC is needed.
The relationship between the clinicopathological features and prognosis of ASC was investigated by both univariate and multivariate analyses. In univariate analyses, pT stage, lymph node metastasis and pTNM stage significantly influenced the survival time. But in multivariate analyses, only lymph node metastasis was found to be the independent risk factors (Table 3). Our finding is consistent with the same result in ASC reported by Zhang et al. (9) and in SCC reported by He et al. (23). The role of adjuvant therapy for esophageal ASC remains unclear. It did not reveal a significant difference in our results and it differed from Chen et al. (8) which found adjuvant therapy to be a better prognostic factor. But Sun et al. (10) reported that patients without postoperative treatment had a longer survival time.
Due to the SCC and AC components both exist in ASC, the prognosis of ASC tended to be SCC or AC remains unclear. Chen et al. (8) compared the prognosis between ASC and different histological grades of SCC and found prognosis of ASC similar to poorly differentiated SCC patients. The TNM staging of ASC is included in esophageal SCC TNM staging system in 7th edition of the AJCC TNM staging system. Whether the esophageal SCC TNM staging system is suitable for ASC also remains unclear. But in our analysis, pT stage, lymph node metastasis and pTNM stage all significantly influenced the prognosis of esophageal ASC patients in Kaplan-Meier analyses. Furthermore, we found lymph node metastasis to be an independent prognostic factor for esophageal ASC according to the multivariate analyses. For pTNM stage is depended on both pT stage and pN stage, the AJCC TNM staging system for esophageal SCC is available for esophageal ASC.
Our study has several limitations, such as retrospective analyses we included, limited sample size and no new adjuvant therapy patients for economic reason in China. These might lead to bias in the result. Larger sample cohort studies are needed to perform in future.
In conclusion, primary ASC of the esophagus is a rare disease with a high misdiagnosis rate by endoscopic biopsy. The prognosis of esophageal ASC is not worse than esophageal SCC and AC. The lymph node metastasis is the only independent risk factor. The TNM staging system of esophageal SCC is applicable for esophageal ASC.
Acknowledgements
None.
Footnote
Conflict of Interests: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of West China Hospital (No.201649).
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Hurst RD. Ch.18.Epidemiology of Small-Bowel Tumors. In: Posner M.C., Vokes E.E., Weichselbaum R.R., editors. Cancer of the Upper Gastrointestinal Tract. Shelton, CT: PMPH-USA Ltd; 2002:336-42.
- Bombí JA, Riverola A, Bordas JM, et al. Adenosquamous carcinoma of the esophagus. A case report. Pathol Res Pract 1991;187:514-9; discussion 519-21. [PubMed]
- Zhang DK, Su XD, Lin P, et al. Clinical analysis of 22 cases of esophageal adenosquamous carcinoma. Zhonghua Zhong Liu Za Zhi 2009;31:302-4. [PubMed]
- Zhang Y, Yang K, Zhang G, et al. Esophageal adenosquamous carcinoma: a case report. Journal of Modern Oncology 2011;19:403-4.
- Francioni F, Tsagkaropoulos S, Telha V, et al. Adenosquamous carcinoma of the esophagogastric junction. Case report. G Chir 2012;33:123-5. [PubMed]
- Chen SB, Weng HR, Wang G, et al. Primary adenosquamous carcinoma of the esophagus. World J Gastroenterol 2013;19:8382-90. [Crossref] [PubMed]
- Zhang HD, Chen CG, Gao YY, et al. Primary esophageal adenosquamous carcinoma: a retrospective analysis of 24 cases. Dis Esophagus 2014;27:783-9. [Crossref] [PubMed]
- Sun YH, Lin SW, Chen CH, et al. Adenosquamous Carcinoma of the Esophagus and Esophagogastric Junction: Clinical Manifestations and Treatment Outcomes. J Gastrointest Surg 2015;19:1216-22. [Crossref] [PubMed]
- Yachida S, Nakanishi Y, Shimoda T, et al. Adenosquamous carcinoma of the esophagus. Clinicopathologic study of 18 cases. Oncology 2004;66:218-25. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Fléjou JF. WHO Classification of digestive tumors: the fourth edition. Ann Pathol 2011;31:S27-31.
- McPEAK E. ARONS WL. Adenoacanthoma of the esophagus; a report of one case with consideration of the tumor's resemblance to so-called salivary gland tumor. Arch Pathol (Chic) 1947;44:385-90. [PubMed]
- Kumagai Y, Ishiguro T, Kuwabara K, et al. Primary mucoepidermoid carcinoma of the esophagus: review of the literature. Esophagus 2014;11:81. [Crossref]
- Lam KY, Loke SL, Ma LT. Histochemistry of mucin secreting components in mucoepidermoid and adenosquamous carcinoma of the oesophagus. J Clin Pathol 1993;46:1011-5. [Crossref] [PubMed]
- Pascal RR, Clearfield HR. Mucoepidermoid (adenosquamous) carcinoma arising in Barrett's esophagus. Dig Dis Sci 1987;32:428-32. [Crossref] [PubMed]
- Zhao S, Xue Q, Ye B, et al. Synchronous primary carcinosarcoma and adenosquamous carcinoma of the esophagus. Ann Thorac Surg 2011;91:926-8. [Crossref] [PubMed]
- Goldstein SR, Yang GY, Curtis SK, et al. Development of esophageal metaplasia and adenocarcinoma in a rat surgical model without the use of a carcinogen. Carcinogenesis 1997;18:2265-70. [Crossref] [PubMed]
- Pera M, Brito MJ, Poulsom R, et al. Duodenal-content reflux esophagitis induces the development of glandular metaplasia and adenosquamous carcinoma in rats. Carcinogenesis 2000;21:1587-91. [Crossref] [PubMed]
- Lam KY, Dickens P, Loke SL, et al. Squamous cell carcinoma of the oesophagus with mucin-secreting component (muco-epidermoid carcinoma and adenosquamous carcinoma): a clinicopathologic study and a review of literature. Eur J Surg Oncol 1994;20:25-31. [PubMed]
- Huang Q, Shi J, Sun Q, et al. Distal esophageal carcinomas in Chinese patients vary widely in histopathology, but adenocarcinomas remain rare. Hum Pathol 2012;43:2138-48. [Crossref] [PubMed]
- He Z, Wu S, Li Q, et al. Use of the metastatic lymph node ratio to evaluate the prognosis of esophageal cancer patients with node metastasis following radical esophagectomy. PLoS One 2013;8:e73446. [Crossref] [PubMed]