Left atrial appendage exclusion for atrial fibrillation: does the protection from stroke prevail in the long-term?
Introduction
On March 13th 2015, the approval of the Watchman left atrial appendage (LAA) closure device (Boston Scientific, Marlborough, MA, USA) by the Food and Drug Administration (FDA) introduced an important tool for stroke prevention for patients with atrial fibrillation (AF) in the United States (1). AF is the most common arrhythmia in clinical practice and afflicts approximately 33.5 million people worldwide (2). Stroke is a feared complication of AF, and systemic anticoagulation is a standard of care for stroke prevention in AF. However, systemic anticoagulation is fraught with potential disadvantages, such as bleeding, need for compliance with medication, compliance with a regular diet in case of warfarin, medication interactions, and need for temporary interruption during surgical procedures.
The Watchman device is a self-expanding nitinol structure with a porous covering that can percutaneously occlude the LAA. Its efficacy for stroke prevention was tested in randomized clinical trials PROTECT AF and PREVAIL (3-5). In the December 2015 edition of JACC Interventions, Wiebe et al. report long-term single center outcomes with the Watchman device (6). Before judging the efficacy of the Watchman device for stroke prevention, it is important to take a step back and understand the etiology of stroke in AF. Is stroke in AF due to thromboembolism from the LAA, or is AF a marker of elevated stroke risk from multiple systemic causes? Local therapy such as appendage exclusion cannot be expected to treat a potentially systemic pathophysiology. In this article we: (I) review the literature implicating the LAA in stroke in AF; (II) summarize the experience with surgical appendage exclusion; (III) discuss the article by Wiebe et al. in context of the PROTECT AF and PREVAIL AF trials; and (IV) provide the reader with a snapshot of future directions in appendage occlusion.
Is AF and stroke an association or causation?
While the association of stroke in patients with AF and rheumatic heart disease, especially mitral stenosis, was widely accepted, the association of non-valvular AF with stroke was established around 30 years ago by the Framingham study (7). The LAA was implicated in the pathogenesis of stroke in non-valvular AF by autopsy data. Davies et al. demonstrated in 1972 that 62% patients with long-term AF had thrombi in the LAA compared to 12% with short-term AF (8). In 1996, Blackshear et al. reviewed 23 studies and reported that thrombi, when present, extended to the left atrial cavity in 10% patients with non-valvular AF compared to 43% of patients with valvular AF (9). A previous autopsy study had also highlighted the difference in anatomical distribution of atrial thrombi between valvular and non-valvular AF patients. Among patients with atrial thrombi, valvular AF patients had left atrial main wall thrombi in 26.5% cases compared to 13.5% cases in the non-valvular AF group (10). This body of literature led to the hypothesis that stasis in the LAA leads to thrombus formation in this location and systemic embolization resulting in stroke.
Two challenges in attributing ischemic strokes in AF to LAA thrombi alone are: (I) patients with absence of left atrial thrombus after a recent stroke and (II) lack of temporal association between AF and stroke. Manning et al. reported absence of LAA thrombus in 57% after recent stroke (11). The possible explanations include embolization of the entire thrombus mass into the brain, thrombolysis from natural causes or anticoagulation, and etiology of stroke other than AF-related embolism. The reality is likely a combination of these explanations. Etiologies of stroke other than embolism are reported in AF. An analysis from SPAF I–III reported 68% strokes in AF were secondary to cardioembolism. Warfarin reduced cardioembolic stroke, while aspirin reduced non-cardioembolic stroke (12). An autopsy study by Yamanouchi et al. is consistent with this observation with 64% cardioembolic strokes in AF patients compared to 3.6% cardioembolic strokes in patients without AF (Figure 1) (13). Thus, stroke in AF is a combination of local causes (LAA thrombosis) and systemic factors. Some strokes that can be prevented by medical therapy might not be prevented by left atrial occlusion strategies.
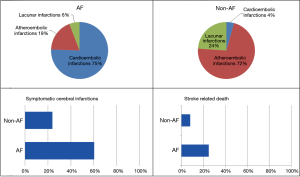
Another confounding question referenced above is the temporal relationship of AF with stroke, or lack thereof. Multiple studies have demonstrated that subclinical AF lasting as little as 6 minutes is a risk factor for stroke (14,15). A substudy from the ASSERT trial reported that only 4/26 (15%) patients had AF in the month prior to the stroke, and only 1/26 was in AF at the time of stroke (16). One explanation is that this study included only patients with >6 minutes of AF and could have missed shorter AF episodes that might predispose to stroke. Another explanation is that atrial rhythm by surface electrocardiogram (ECG) is a poor predictor of left atrial mechanical function as assessed by Doppler echocardiography. Warraich et al. reported one fourth of patients with paroxysmal AF had evidence of low LAA ejection velocity even when surface ECG showed sinus rhythm (17). Although temporal association of AF and stroke is unclear, studies are limited by current investigative modalities in terms of detection of brief episodes of AF and poor LAA function despite sinus rhythm on surface ECG.
Surgical LAA exclusion
Recognition of the LAA as a nidus of thrombus formation in non-valvular AF patients led to the practice of appendage ligation and excision in patients undergoing cardiac surgery. Retrospective studies have reported reduction in stroke after complete LAA ligation (18,19). Randomized data regarding efficacy of surgical appendage ligation or excision are lacking. A small randomized pilot study, LAAOS II, reported 1/25 strokes in patients with occlusion compared to 3/25 without occlusion (20). A large RCT (LAAOS III) is currently enrolling 4,700 patients to answer this question, and results are expected in 2020 (21).
The inability to completely exclude or excise the LAA is the Achilles heel of surgical removal of the LAA. In the study by García-Fernández et al., risk of embolic events actually increased in patients with incomplete appendage ligation (18). Another small study reported a 22% risk of embolic events at follow-up in patients with incomplete appendage ligation (22). Incomplete occlusion might increase stroke risk by impending flow of blood resulting in stasis. As many as 36–100% patients may have incomplete surgical LAA exclusion, and surgical technique and operator experience both have a major impact on the ability to completely exclude the LAA (22,23).
PROTECT AF, PREVAIL AF and study by Wiebe et al.
The efficacy of the Watchman device for stroke prevention in AF was assessed by the PROTECT AF and PREVAIL trials. The PROTECT AF trial, published in 2009, included 707 patients randomly assigned in a 2:1 ratio to percutaneous appendage closure with the Watchman device or warfarin (4). Percutaneous appendage closure was non-inferior to warfarin, with a primary efficacy rate (stroke, cardiovascular death, systemic embolism) of 3.0 per 100 patient-years in the intervention arm and 4.9 per 100 patient-years in the control arm. Primary safety events including major bleeding, hemorrhagic stroke, pericardial effusion, and procedure-related ischemic stroke were more common in the intervention arm (7.4 per 100 patient-years vs. 4.4 per 100 patient-years). A notable finding in the PROTECT AF study is the high rate of intracerebral hemorrhage in the warfarin arm compared to contemporary trials of anticoagulation with novel oral anticoagulants (NOACs) (Figure 2).
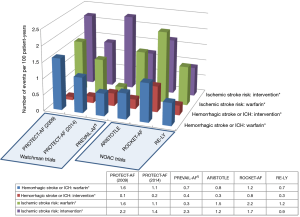
Due to concerns raised by the FDA related to acute safety events in the PROTECT AF trial, the PREVAIL trial was designed collaboratively with the FDA by the study sponsor and published in 2014 (3). Enrolling 407 patients in a 2:1 ratio to intervention and control arms, this study failed to demonstrate statistical noninferiority of percutaneous appendage closure. The 18-month rate ratio of primary efficacy endpoint for the intervention to control arm was 1.07, with 95% upper credible interval 0.57 to 1.89, which exceeded the pre-specified noninferiority margin of 1.75. However, the study met the noninferiority criteria for the late-ischemic primary efficacy endpoint (stroke or systemic embolism >7 days after randomization) and the early primary safety endpoint for the intervention arm (6/269 safety events).
Around the same time as the results of PREVAIL were published, long-term follow-up of PROTECT AF were reported (5). After mean 2.3±1.1 years of follow-up, the primary efficacy event rates were 3.0% vs. 4.3% per 100 patient-years for the intervention vs. control arm, which met the noninferiority criteria. There were numerically more primary safety events in the intervention arm (5.5% vs. 3.6% per year; relative risk 1.52; 95% confidence interval 0.95–2.70).
On the basis of the results of the PREVAIL and the long-term follow-up data from PROTECT AF, an FDA panel voted 13:1 in December 2013 that the intervention is safe, effective, and that the benefits of the intervention exceed the risks in the enrolled trial population (24). However, the results available to the panel and published in PREVAIL AF were locked in January 2013. The sponsor updated the PREVAIL AF data in June 2014. There were 13 additional ischemic strokes in the intervention arm compared with one in the control arm. The intervention no longer met the noninferiority criteria for the primary efficacy endpoint, even after including long-term follow-up from the PROTECT AF cohort. Hence, another FDA panel meeting was convened in 2014. The FDA voted 12 to 0 that the intervention is safe, 6 to 7 that it is not effective and 6 to 5 (with one member abstaining) that its benefits outweigh the risk (24). Overall, the panel suggested that the device has a role as second line therapy to anticoagulation for stroke prevention in appropriately selected AF patients. Ultimately, the FDA approved the Watchman device in 2015 for patients with non-valvular AF with elevated risk of stroke based on CHADS2 or CHADS2-VASc scores who are eligible for warfarin but have an appropriate rationale for a nonpharmacological alternative to warfarin (1).
Lack of long-term outcomes is a valid concern with the Watchman device given the increase in ischemic strokes upon follow-up of the PREVAIL cohort. The study by Wiebe et al. in 2015 in JACC Interventions is a timely study addressing these concerns (6). The authors reported results up to 5 years follow-up from a cohort of 96 patients who underwent percutaneous appendage closure using the Watchman device at a single center. Over mean 3.0±1.6 years of follow-up, stroke/transient ischemic attack (TIA), intracranial hemorrhage (ICH), and death occurred in 1.4%, 1.1%, and 3.5% of the trial population respectively. Among the four patients with ischemic stroke/TIA at follow-up, three events occurred more than 1 year after device implantation. Overall, these data compare favorably to the results of the PROTECT AF and PREVAIL trial (Table 1). There continues to be concerns regarding the long-term efficacy of the Watchman device with regards to ischemic stroke prevention, and results of the post-approval studies mandated by the FDA will be valuable in informing patients and providers.
Weighing the risks and benefits of stroke prevention strategies in AF
The success of any therapy must be judged by assessing the risks and benefits of the therapy against the alternatives.
Anticoagulation vs. appendage exclusion
Patient compliance and the ability to tolerate long-term medical therapy are major barriers to long-term systemic anticoagulation in patients receiving warfarin or NOACs. Less than 50% of patients with risk factors for stroke and AF are prescribed or fill prescriptions for warfarin after AF presentation (28,29). Even if treatment is initiated, 40% of patients cease to use warfarin at 4-year follow-up (30). Warfarin is inconvenient to patients due to the need for regular international normalized ratio (INR) monitoring, interactions with medications, and diet. Only 60% of patients have an INR in the target range of 2.0 to 3.0, even in closely monitored clinical trial settings (31). NOACs offer more convenience compared to warfarin; however, NOACs also need to be stopped for major surgeries and bleeding episodes. Appendage exclusion will continue to provide benefit in these circumstances while systemic therapy may not.
Surgical vs. endocardial vs. epicardial appendage exclusion
The LAA can be excluded in a variety of methods including surgical methods discussed previously and minimally invasive epicardial and percutaneous endocardial methods. Some of the ischemic stroke risk in endocardial occlusion is from thrombus formation on the device. For this reason, warfarin and clopidogrel are recommended for 3–6 months after endocardial LAA occlusion. Epicardial devices have the benefit of avoiding this issue. There is cessation of LAA electrical activity after epicardial ligation, but it is unclear whether this translates to a reduction in AF burden (32,33). However, epicardial devices require pericardial access, which is a difficult skill to master. Additionally, randomized controlled trials supporting the role of epicardial ligation are lacking compared to endocardial occlusion (Table 2).

Full table
Future directions in appendage exclusion
Apart from the Watchman device, there are several other epicardial and endocardial LAA exclusion devices under development (34). The LARIAT system offers a hybrid and endocardial and epicardial approach to LAA ligation and was effective in appendage ligation in observational studies (35,36). The Aegis system is a completely intrapericardial ligation approach utilizing a grabber with embedded electrodes to recognize LAA signals and deliver a preformed suture to ligate the appendage. This approach is feasible in humans, and larger randomized trials are awaited (37). Other surgical epicardial ligation approaches under development include the AtriClip Pro and the Tigerpaw system II, which are feasible according to first-in-human studies; further clinical trials are awaited (38,39).
The Plaato device was the first device designed specifically for endocardial appendage exclusion but is no longer under development due to financial considerations (40). Small retrospective studies support the efficacy and safety of the Amplatzer cardiac plug for appendage exclusion (41-44). However, randomized data are not available, and a randomized clinical trial was designed but could not be conducted due to failure to obtain the investigational device exemption from the FDA (45). A percutaneously delivered transcatheter patch utilizing surgical adhesives was effective in atrial appendage exclusion, but further studies are not available (46). An animal study has demonstrated feasibility of appendage exclusion with the LAmbre device (47).
Conclusions
Similar to several clinical conundrums in medicine, there is no “one-size-fits-all” approach for stroke prevention in AF. Patient characteristics, preferences, cost considerations, and provider expertise must all be taken into account. What is clear is that AF predisposes to strokes that are larger, more disabling, and deadlier than strokes from other causes. Prevention by either anticoagulation or LAA exclusion is essential.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Yue Liu (Associate professor, Department of Cardiology, The First Affiliated Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Food and Drug Administration. Premarket approval application (PMA) for the WATCHMAN LAA Closure Technology. 2016. Available online: http://101.96.10.42/www.accessdata.fda.gov/cdrh_docs/pdf13/p130013a.pdf
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837-47. [Crossref] [PubMed]
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1-12. [Crossref] [PubMed]
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534-42. [Crossref] [PubMed]
- Reddy VY, Doshi SK, Sievert H, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation 2013;127:720-9. [Crossref] [PubMed]
- Wiebe J, Franke J, Lehn K, et al. Percutaneous Left Atrial Appendage Closure With the Watchman Device: Long-Term Results Up to 5 Years. JACC Cardiovasc Interv 2015;8:1915-21. [Crossref] [PubMed]
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med 1987;147:1561-4. [Crossref] [PubMed]
- Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J 1972;34:520-5. [Crossref] [PubMed]
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755-9. [Crossref] [PubMed]
- Aberg H. Atrial fibrillation. I. A study of atrial thrombosis and systemic embolism in a necropsy material. Acta Med Scand 1969;185:373-9. [Crossref] [PubMed]
- Manning WJ, Silverman DI, Waksmonski CA, et al. Prevalence of residual left atrial thrombi among patients with acute thromboembolism and newly recognized atrial fibrillation. Arch Intern Med 1995;155:2193-8. [Crossref] [PubMed]
- Hart RG, Pearce LA, Miller VT, et al. Cardioembolic vs. noncardioembolic strokes in atrial fibrillation: frequency and effect of antithrombotic agents in the stroke prevention in atrial fibrillation studies. Cerebrovasc Dis 2000;10:39-43. [Crossref] [PubMed]
- Yamanouchi H, Nagura H, Mizutani T, et al. Embolic brain infarction in nonrheumatic atrial fibrillation: a clinicopathologic study in the elderly. Neurology 1997;48:1593-7. [Crossref] [PubMed]
- Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120-9. [Crossref] [PubMed]
- Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478-86. [Crossref] [PubMed]
- Brambatti M, Connolly SJ, Gold MR, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014;129:2094-9. [Crossref] [PubMed]
- Warraich HJ, Gandhavadi M, Manning WJ. Mechanical discordance of the left atrium and appendage: a novel mechanism of stroke in paroxysmal atrial fibrillation. Stroke 2014;45:1481-4. [Crossref] [PubMed]
- García-Fernández MA, Pérez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003;42:1253-8. [Crossref] [PubMed]
- Kim R, Baumgartner N, Clements J. Routine left atrial appendage ligation during cardiac surgery may prevent postoperative atrial fibrillation-related cerebrovascular accident. J Thorac Cardiovasc Surg 2013;145:582-9; discussion 589. [Crossref] [PubMed]
- Whitlock RP, Vincent J, Blackall MH, et al. Left Atrial Appendage Occlusion Study II (LAAOS II). Can J Cardiol 2013;29:1443-7. [Crossref] [PubMed]
- Whitlock R, Healey J, Vincent J, et al. Rationale and design of the Left Atrial Appendage Occlusion Study (LAAOS) III. Ann Cardiothorac Surg 2014;3:45-54. [PubMed]
- Katz ES, Tsiamtsiouris T, Applebaum RM, et al. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J Am Coll Cardiol 2000;36:468-71. [Crossref] [PubMed]
- Kanderian AS, Gillinov AM, Pettersson GB, et al. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol 2008;52:924-9. [Crossref] [PubMed]
- Waksman R, Pendyala LK. Overview of the Food and Drug Administration circulatory system devices panel meetings on WATCHMAN left atrial appendage closure therapy. Am J Cardiol 2015;115:378-84. [Crossref] [PubMed]
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. [Crossref] [PubMed]
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. [Crossref] [PubMed]
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [Crossref] [PubMed]
- Brass LM, Krumholz HM, Scinto JM, et al. Warfarin use among patients with atrial fibrillation. Stroke 1997;28:2382-9. [Crossref] [PubMed]
- Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med 1999;131:927-34. [Crossref] [PubMed]
- Gallagher AM, Rietbrock S, Plumb J, et al. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thromb Haemost 2008;6:1500-6. [Crossref] [PubMed]
- Holmes DR Jr, Lakkireddy DR, Whitlock RP, et al. Left atrial appendage occlusion: opportunities and challenges. J Am Coll Cardiol 2014;63:291-8. [Crossref] [PubMed]
- Han FT, Bartus K, Lakkireddy D, et al. The effects of LAA ligation on LAA electrical activity. Heart Rhythm 2014;11:864-70. [Crossref] [PubMed]
- Syed FF, Rangu V, Bruce CJ, et al. Percutaneous ligation of the left atrial appendage results in atrial electrical substrate modification. Transl Res 2015;165:365-73. [Crossref] [PubMed]
- Syed FF, Noheria A, DeSimone CV, et al. Left Atrial Appendage Ligation and Exclusion Technology in the Incubator. J Atr Fibrillation 2015;8:61-70. [PubMed]
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62:108-18. [Crossref] [PubMed]
- Stone D, Byrne T, Pershad A. Early results with the LARIAT device for left atrial appendage exclusion in patients with atrial fibrillation at high risk for stroke and anticoagulation. Catheter Cardiovasc Interv 2015;86:121-7. [Crossref] [PubMed]
- Bruce CJ, Asirvatham SJ, McCaw T, et al. Novel percutaneous left atrial appendage closure. Cardiovasc Revasc Med 2013;14:164-7. [Crossref] [PubMed]
- Slater AD, Tatooles AJ, Coffey A, et al. Prospective clinical study of a novel left atrial appendage occlusion device. Ann Thorac Surg 2012;93:2035-8; discussion 2038-40. [Crossref] [PubMed]
- Emmert MY, Puippe G, Baumüller S, et al. Safe, effective and durable epicardial left atrial appendage clip occlusion in patients with atrial fibrillation undergoing cardiac surgery: first long-term results from a prospective device trial. Eur J Cardiothorac Surg 2014;45:126-31. [Crossref] [PubMed]
- Bayard YL, Omran H, Neuzil P, et al. PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: results from the European PLAATO study. EuroIntervention 2010;6:220-6. [Crossref] [PubMed]
- Park JW, Bethencourt A, Sievert H, et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv 2011;77:700-6. [Crossref] [PubMed]
- Urena M, Rodés-Cabau J, Freixa X, et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol 2013;62:96-102. [Crossref] [PubMed]
- López-Mínguez JR, Eldoayen-Gragera J, González-Fernández R, et al. Immediate and one-year results in 35 consecutive patients after closure of left atrial appendage with the Amplatzer cardiac plug. Rev Esp Cardiol (Engl Ed) 2013;66:90-7. [Crossref] [PubMed]
- Guérios EE, Schmid M, Gloekler S, et al. Left atrial appendage closure with the Amplatzer cardiac plug in patients with atrial fibrillation. Arq Bras Cardiol 2012;98:528-36. [PubMed]
- Clinicaltrials.gov. Trial of device that is not approved or cleared by the U.S. FDA. Available online: https://clinicaltrials.gov/ct2/show/NCT01118299?term=NCT011
- Toumanides S, Sideris EB, Agricola T, et al. Transcatheter patch occlusion of the left atrial appendage using surgical adhesives in high-risk patients with atrial fibrillation. J Am Coll Cardiol 2011;58:2236-40. [Crossref] [PubMed]
- Lam YY. A new left atrial appendage occluder (Lifetech LAmbre Device) for stroke prevention in atrial fibrillation. Cardiovasc Revasc Med 2013;14:134-6. [Crossref] [PubMed]



