“The scaffolding must be removed once the house is built”—spontaneous coronary artery dissection and the potential of bioresorbable scaffolds
Spontaneous coronary artery dissection (SCAD) is defined as the separation in any of the three layers of the coronary artery wall that is not iatrogenic or caused by trauma (1). Although SCAD is often asymptomatic, it is increasingly recognized as the underlying etiology in acute coronary syndromes (ACS) (2). SCAD mainly affects women (>90% of cases), most commonly between 44 to 55 years-of-age (3-6). SCAD may result from an intimal disruption or “tear” with formation of an intramural hematoma (IMH), or spontaneous intramural bleeding, likely due to the rupture of vasa vasorum (7). SCAD resulting from either mechanism results in blood accumulation within the newly formed false lumen, which may compress the true lumen to varying degrees (1), thus presenting as myocardial ischemia, ACS, cardiogenic shock or sudden cardiac death (4,8,9). SCAD may extensively propagate in anterograde and/or retrograde fashion, with the mean length of dissection typically >45 mm on quantitative coronary angiography (10).
SCAD occurs in the presence of a predisposing arteriopathy, which when combined with a precipitating factor, results in the dissection of the coronary artery wall (1). The most common associated arteriopathy is fibromuscular dysplasia (FMD) (3,4), which is characterized by dysplasia, disorganization, and loss of smooth muscle cells, fibroblasts and connective tissue that may affect any of the three arterial layers and elastic laminae (11-13), therefore predisposing the affected arteries to dissection and aneurysm formation. FMD may manifest as coronary tortuosity, dilatation or ectasia (10), and as a “string of beads” appearance (stenosis alternating with dilatation) in non-coronary vasculature (3). Pregnancy is another associated predisposing factor, likely due to effects of high progesterone levels in weakening the arterial media through alterations in the elastic fiber and mucopolysaccharide content as well as decreasing collagen synthesis (14). Multiple pregnancies can lead to repetitive impairment of arterial wall integrity and a higher risk for SCAD (4,14) (Figure 1). Similarly, long-term exposure to progesterone by hormonal replacement therapy may increase the risk of SCAD (15). Other less common predisposing factors are connective tissue diseases (e.g., Marfan and Ehlers-Danlos type 4 syndromes) (16) or chronic systemic inflammatory diseases that are associated with vasculitis (17). Precipitating factors either lead to a Valsalva-like increase in the intrathoracic pressure that can be transmitted to coronary arteries as shear stress, or may raise catecholamine levels and thus result in increased vascular shear stress (1,4). The increased shear stress may then trigger architectural disruption or spontaneous intramural bleeding (4). Likely precipitating factors include intense emotional stress (more frequently reported in women) (4), physical activities (especially isometric exercises, more frequently reported in men) (18), sympathomimetic drugs [including illicit drugs such as cocaine (19,20)], and intense activities inducing Valsalva-like maneuvers (e.g., childbirth, coughing, vomiting and bowel movement) (4,15).
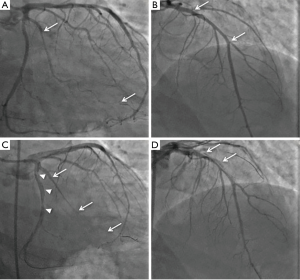
On angiography, SCAD may appear as contrast staining in the arterial wall associated with multiple radiolucent lumens (type 1), diffuse stenosis of varying length and severity with abrupt change in the arterial caliber from the normal diameter to diffuse narrowing (type 2, most common appearance, usually >20 mm), or focal/tubular stenosis mimicking atherosclerosis (type 3, usually <20 mm) (1). Thus SCAD may be difficult to recognize angiographically, requiring a high index of suspicion for its presence (especially in ACS in younger patients). SCAD most commonly involves the left anterior descending (LAD) artery and its branches, followed in incidence by circumflex and right coronary arteries and their branches (4,5,8). Most dissections involve the mid to distal segments, with <10% affecting the proximal coronary arteries or left main coronary artery (4). Thrombolysis In Myocardial Infarction (TIMI) flow grades 0, 1, 2 and 3 are reported in ~25%, ~10%, ~15% and ~50% of patients with SCAD respectively (4,5).
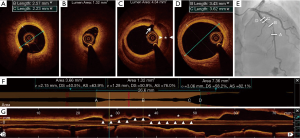
Diagnosis of type 2 or 3 SCAD often requires intravascular imaging by intravascular ultrasound (IVUS) or optical coherence tomography (OCT) after administration of intracoronary vasodilators to exclude vasospasm (1). Both OCT and IVUS can provide additional information on the presence of IMH or true and false lumens. OCT has superior axial resolution compared to IVUS and thus provides better visualization of intimal tears, intraluminal thrombi, false lumen, and IMH (Figure 2). However, due to limited depth penetration, OCT may not depict the entire depth of the IMH (21,22); IVUS is superior in this regard (23). It is critical to note the potential risks with intracoronary imaging in the setting of SCAD, including the potential to extend the dissection with the guide wire or imaging catheter, guide catheter-induced iatrogenic dissection, hydraulic extension by contrast injection required in OCT, and imaging catheter-induced coronary occlusion (1). Therefore, intracoronary imaging in the context of the suspected SCAD should be performed only if required for diagnosis or to guide treatment, using careful meticulous techniques.
In the absence of data from randomized clinical trials, management of SCAD is largely based on the results from observational series. Since the majority of dissections spontaneously heal within ~6 months (22), and because revascularization of SCAD is associated with high failure rates, the mainstay of treatment is conservative medical therapy (24). Beta blockers (to reduce coronary shear stress) and anti-platelet agents (to reduce thrombosis) are the main pharmacotherapies for SCAD (1). Other potentially beneficial agents such as vasodilators (if spasm is suspected as a precipitating factor), angiotensin-converting enzymes [especially if left ventricular function is abnormal (25)], and lipid-lowering therapy [e.g., statins in the presence of dyslipidemia for secondary prevention (5)] may be considered as indicated. Since SCAD may progress in up to 10% of patients that are conservatively managed thus necessitating revascularization (26), inpatient monitoring is recommended depending on the symptoms and anatomical location of SCAD (1).
A primary revascularization approach may be necessary in patients with SCAD with dissection involving the left main coronary artery, compromised coronary flow on angiography, ongoing or recurrent ischemia, hemodynamic instability, or ventricular arrhythmias (1). Urgent coronary artery bypass graft surgery (CABG) may be considered for patients with left main dissections, extensive dissections involving proximal arteries, or in patients in whom percutaneous coronary intervention (PCI) has failed or is not anatomically suitable (4,8,26). PCI in SCAD is challenging. Arteries with SCAD are susceptible to iatrogenic dissection and extension (Figure 1), entering the true lumen with guide wires in type 1 dissection may be difficult, and balloon angioplasty and stenting may propagate the IMH proximally or distally (1). Long stents are often required to cover the entire length of SCAD—thus increasing the risk of stent thrombosis—and dissections mostly involve distal coronary segments, which may be too small in diameter for stenting. Moreover, absorption of the IMH over time may result in late acquired malposition (27), which after PCI performed on atherosclerotic lesions has been associated with stent thrombosis (28). It is therefore not surprising that the outcomes of PCI in SCAD have been suboptimal. Failure of PCI has been reported in 35–50% of cases, with extension of dissection occurring in 57%, and urgent CABG needed in 9–13% (4,8,26). Furthermore, relatively high rates of stent thrombosis have been observed (~6%) (4,8).
Meticulous technique and careful strategies are therefore needed when considering PCI to treat SCAD. PCI through femoral access should be the preferred route as higher iatrogenic dissection (~3-fold) has been reported with radial approach (4). Intravascular imaging with OCT or IVUS can guide entry to the true lumen and optimization of stent deployment. Long stents (5 to 10 mm longer on both edges of the IMH) are typically needed to ensure sealing IMH propagation caused by compression during stent deployment (1). For longer lesions that require multiple stents, a multistep approach starting by stenting the distal edge, followed by the proximal edge, and finished by stenting the middle segment may be useful in preventing extension of the IMH (29).
The use of bioresorbable vascular scaffolds (BVS) for revascularization in SCAD is an intuitively attractive option (30-32). The German philosopher Friedrich Nietzsche once noted: “The scaffolding must be removed once the house is built” (33). BVS provide a temporary scaffold to re-establish coronary flow in arteries affected by SCAD, with scaffold resorption over time avoiding a lifetime commitment to permanent metallic stents in the typically young patient with SCAD. The ideal scaffold for treatment of SCAD should resorb within 6–12 months as the dissection heals, rather than the longer durations (2–3 years) with currently available BVS technology, especially since—unlike atherosclerotic lesions—sustained radial strength of the scaffold may not be necessary for treatment of SCAD. Nevertheless, as has been shown with PCI in atherosclerotic lesions with BVS (34), meticulous technique is required to optimize temporary scaffolding by BVS in order to achieve acute procedural and long-term results that are comparable to the latest generations of metallic drug-eluting stents. Careful strategies for deployment of BVS in SCAD are needed to take advantage of the unique characteristics of the bioresorbable scaffolds while avoiding suboptimal outcomes. Guidance by intravascular imaging is strongly encouraged—taking into account the potential risks aforementioned—which allows for detailed delineation of SCAD, appropriate scaffold sizing, visualization of scaffolds and their performance (expansion, apposition), vascular wall response to scaffolds and changes in the appearance of the struts over time (35). In theory, the typically long length of BVS required to treat SCAD may increase the risk of scaffold restenosis or thrombosis due to the need for overlap. Nevertheless, most vessels affected by SCAD are free of significant atherosclerosis (1), implying ease of scaffold expansion and lower risk of new edge lesions due to neointimal hyperplasia or constrictive remodeling that may occur at the sites of untreated atherosclerosis in the reference segments after PCI (36). Moreover, neoatherosclerosis—a mechanism for late stent failure that correlates with atherosclerosis progression in native coronary arteries (37)—may be less of an issue in the relatively young population of patients with SCAD treated with BVS. Since data from BVS deployment for native atherosclerotic lesions have shown a propensity for increased scaffold thrombosis in small arteries (<2.5 mm in diameter) (38), BVS deployment for SCAD in arteries of this size may need to be avoided with consideration of natural vessel healing following proximal vessel treatment. There have been case reports of using cutting balloons to fenestrate the IMH to allow for decompression of the false lumen into the true lumen (39,40). This approach may also prevent propagation of the IMH if stenting is subsequently required (40). Nevertheless, due to a theoretical risk of coronary rupture in the setting of SCAD, cautious technique with the use of undersized cutting balloons should be undertaken (1).
Life style modifications are necessary after initial treatment of SCAD. Dedicated programs that include rehabilitation exercise—aiming for low target heart rate and systolic blood pressure—and psychosocial counseling have been shown to be beneficial in patients who have suffered SCAD (1). In general, patients are advised to avoid lifting weights [>20 pounds in women, >50 pounds in men (18)], although whether these restrictions will reduce the risk of recurrent SCAD is unknown (1). Other predisposing factors such as hormonal therapy should generally be avoided and women of childbearing age should be counselled on future pregnancies, which likely need to be avoided because of the relatively high risk of recurrent SCAD [e.g., ~13% in a small series (41)].
In conclusion, SCAD is a relatively uncommon cause of ACS, most frequently found in young to middle aged women, the occurrence of which has been increasingly appreciated in recent times. Combinations of precipitating stressors in the presence of underlying predisposing factors constitute the triggering mechanism for both intimal disruption and formation of the IMH. Conservative medical therapy and life style modifications remain the mainstay of management, with revascularization by PCI or urgent CABG reserved for unstable patients. PCI using BVS has potential advantages compared to metallic stents in relatively young patients with SCAD, and has been successfully used in isolated case reports. As randomized trials in this condition are unlikely, larger series with long-term outcomes are required to establish whether intravascular imaging-guided BVS deployment is a safe and efficacious therapy in the subset of patients with SCAD.
Acknowledgements
None.
Footnote
Provenance: This is an invited Commentary commissioned by the Section Editor Xiaoyan Wang (Phd student in Cardiology, Fudan University Shanghai Medical College, Shanghai, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Saw J, Mancini GB, Humphries KH, et al. Contemporary Review on Spontaneous Coronary Artery Dissection. J Am Coll Cardiol 2016;68:297-312. [Crossref] [PubMed]
- Tweet MS, Gulati R, Aase LA, et al. Spontaneous coronary artery dissection: a disease-specific, social networking community-initiated study. Mayo Clin Proc 2011;86:845-50. [Crossref] [PubMed]
- Saw J, Ricci D, Starovoytov A, et al. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013;6:44-52. [Crossref] [PubMed]
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645-55. [Crossref] [PubMed]
- Rogowski S, Maeder MT, Weilenmann D, et al. Spontaneous Coronary Artery Dissection: Angiographic Follow-Up and Long-Term Clinical Outcome in a Predominantly Medically Treated Population. Catheter Cardiovasc Interv 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Roura G, Ariza-Solé A, Rodriguez-Caballero IF, et al. Noninvasive Follow-Up of Patients With Spontaneous Coronary Artery Dissection With CT Angiography. JACC Cardiovasc Imaging 2016;9:896-7. [Crossref] [PubMed]
- Maehara A, Mintz GS, Castagna MT, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol 2002;89:466-8. [Crossref] [PubMed]
- Lettieri C, Zavalloni D, Rossini R, et al. Management and Long-Term Prognosis of Spontaneous Coronary Artery Dissection. Am J Cardiol 2015;116:66-73. [Crossref] [PubMed]
- Hill SF, Sheppard MN. Non-atherosclerotic coronary artery disease associated with sudden cardiac death. Heart 2010;96:1119-25. [Crossref] [PubMed]
- Saw J, Mancini GB, Humphries K, et al. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv 2016;87:E54-61. [Crossref] [PubMed]
- Lie JT, Berg KK. Isolated fibromuscular dysplasia of the coronary arteries with spontaneous dissection and myocardial infarction. Hum Pathol 1987;18:654-6. [Crossref] [PubMed]
- Mather PJ, Hansen CL, Goldman B, et al. Postpartum multivessel coronary dissection. J Heart Lung Transplant 1994;13:533-7. [PubMed]
- Brodsky SV, Ramaswamy G, Chander P, et al. Ruptured cerebral aneurysm and acute coronary artery dissection in the setting of multivascular fibromuscular dysplasia: a case report. Angiology 2007;58:764-7. [Crossref] [PubMed]
- Koller PT, Cliffe CM, Ridley DJ. Immunosuppressive therapy for peripartum-type spontaneous coronary artery dissection: case report and review. Clin Cardiol 1998;21:40-6. [Crossref] [PubMed]
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579-88. [Crossref] [PubMed]
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015;115:1672-7. [Crossref] [PubMed]
- Kamran M, Guptan A, Bogal M. Spontaneous coronary artery dissection: case series and review. J Invasive Cardiol 2008;20:553-9. [PubMed]
- Fahmy P, Prakash R, Starovoytov A, et al. Pre-Disposing and Precipitating Factors in Men With Spontaneous Coronary Artery Dissection. JACC Cardiovasc Interv 2016;9:866-8. [Crossref] [PubMed]
- Jaffe BD, Broderick TM, Leier CV. Cocaine-induced coronary-artery dissection. N Engl J Med 1994;330:510-1. [Crossref] [PubMed]
- Ijsselmuiden A, Verheye S. Cocaine-induced coronary artery dissection. JACC Cardiovasc Interv 2009;2:1031. [Crossref] [PubMed]
- Alfonso F, Paulo M, Gonzalo N, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol 2012;59:1073-9. [Crossref] [PubMed]
- Cade J, Mintz GS, Silva RM. Spontaneous coronary artery dissection and healing documented by optical coherence tomography. Einstein (Sao Paulo) 2016;14:435-6. [Crossref] [PubMed]
- Paulo M, Sandoval J, Lennie V, et al. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging 2013;6:830-2. [Crossref] [PubMed]
- Mehta LS, Beckie TM, DeVon HA, et al. Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation 2016;133:916-47. [Crossref] [PubMed]
- Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569-619.
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014;7:777-86. [Crossref] [PubMed]
- Lempereur M, Fung A, Saw J. Stent mal-apposition with resorption of intramural hematoma with spontaneous coronary artery dissection. Cardiovasc Diagn Ther 2015;5:323-9. [PubMed]
- Mintz GS. Why are we so concerned with acute incomplete stent apposition? Eur Heart J Cardiovasc Imaging 2015;16:110-1. [Crossref] [PubMed]
- Walsh SJ, Jokhi PP, Saw J. Successful percutaneous management of coronary dissection and extensive intramural haematoma associated with ST elevation MI. Acute Card Care 2008;10:231-3. [Crossref] [PubMed]
- Cockburn J, Yan W, Bhindi R, et al. Spontaneous coronary artery dissection treated with bioresorbable vascular scaffolds guided by optical coherence tomography. Can J Cardiol 2014;30:1461.e1-3. [Crossref] [PubMed]
- Sengottuvelu G, Rajendran R. Full polymer jacketing for long-segment spontaneous coronary artery dissection using bioresorbable vascular scaffolds. JACC Cardiovasc Interv 2014;7:820-1. [Crossref] [PubMed]
- Sengottuvelu G, Rajendran R, Majumdar D. Capping spontaneous coronary artery dissection with overlapping bioabsorbable scaffolds. Heart Lung Circ 2015;24:e39-40. [Crossref] [PubMed]
- Nietzsche F. The Wanderer and His Shadow, aphorism 335, "Moral for House-Builders" (1880). In: Colli G, Montinari M. editors. Sämtliche Werke: Kritische Studienausgabe. Berlin: de Gruyter, 1980;2:698.
- Ellis SG, Kereiakes DJ, Metzger DC, et al. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N Engl J Med 2015;373:1905-15. [Crossref] [PubMed]
- Karanasos A, Simsek C, Gnanadesigan M, et al. OCT assessment of the long-term vascular healing response 5 years after everolimus-eluting bioresorbable vascular scaffold. J Am Coll Cardiol 2014;64:2343-56. [Crossref] [PubMed]
- Farooq V, Gogas BD, Serruys PW. Restenosis: delineating the numerous causes of drug-eluting stent restenosis. Circ Cardiovasc Interv 2011;4:195-205. [Crossref] [PubMed]
- Kim C, Kim BK, Lee SY, et al. Incidence, clinical presentation, and predictors of early neoatherosclerosis after drug-eluting stent implantation. Am Heart J 2015;170:591-7. [Crossref] [PubMed]
- Stone GW, Granada JF. Very Late Thrombosis After Bioresorbable Scaffolds: Cause for Concern? J Am Coll Cardiol 2015;66:1915-7. [Crossref] [PubMed]
- Yumoto K, Sasaki H, Aoki H, et al. Successful treatment of spontaneous coronary artery dissection with cutting balloon angioplasty as evaluated with optical coherence tomography. JACC Cardiovasc Interv 2014;7:817-9. [Crossref] [PubMed]
- Alkhouli M, Cole M, Ling FS. Coronary artery fenestration prior to stenting in spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2016;88:E23-7. [Crossref] [PubMed]
- Tweet MS, Hayes SN, Gulati R, et al. Pregnancy after spontaneous coronary artery dissection: a case series. Ann Intern Med 2015;162:598-600. [Crossref] [PubMed]


