The impact of smoking status on radiologic tumor progression patterns and response to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors in lung adenocarcinoma with activating EGFR mutations
Introduction
Although smoking tobacco has been shown to be one of the predominant risk factors for lung cancer, 25% of global lung cancer cases are not attributed to tobacco smoke (1). Lung cancer in those who have never smoked is considered to be a distinct disease with unique clinical features (1,2). Female Asian patients have a higher incidence of non-small-cell lung cancer (NSCLC) among never-smokers (2-4). Never-smokers have demonstrated better therapeutic responses to epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) (5-7), probably due to the higher occurrence rate of EGFR mutations in never-smoker groups (5,8-10).
Approximately 90% of clinically relevant EGFR mutations in patients with NSCLC are exon 19 in-frame deletions (exon 19 del) and exon 21 L858R substitutions (exon 21 L858R) (11,12). NSCLC harboring exon 19 del or exon 21 L858R is highly sensitive to EGFR-TKI therapies, such as erlotinib or gefitinib, with response rates ranging from 60–90% and progression free survival (PFS) of 7–13 months (13,14). Afatinib, another clinically available EGFR-TKI, has maximal survival benefit in patients with exon 19 del (15). The frequency of activating EGFR mutations in exon 19 del and exon 21 L858R has been reported to be 40–60% in non-smoking patients compared to 10–20% in smoking-related NSCLC (5,8-10). Moreover, a previous study identified an inverse relationship between incidence of EGFR mutations and number of pack-years of cigarette smoking, with fewer mutations found in patients with longer smoking history (12). Together, these findings suggest that favorable outcomes in response to EGFR-TKI therapy in non-smoking NSCLC patients are due to their higher rate of EGFR-mutation compared with that of smoking patients (16,17).
Few studies have focused directly on smoking history and its relationship with EGFR-TKI therapy outcome in patients with EGFR-mutant NSCLC, and the existing studies have yielded controversial results (12,14,17-20). Several studies showed no significant differences in survival outcomes after EGFR-TKI therapy in lung adenocarcinoma patients who were former/current smokers and those who had never smoked (12,18-20). This further indicates that it is probably the mutation status and not clinical characteristics such as smoking history that underlies clinical outcomes after EGFR-TKI therapy (12,18-20). Recent preclinical studies, however, have shown that smoking aberrantly activates the EGFR pathway, and cells that are activated by smoking are resistant to EGFR-TKIs (21,22). One recent retrospective study showed that more than 30 pack-years of smoking is an independent negative predictive factor of EGFR-TKI therapy outcome in lung adenocarcinoma patients with EGFR-activating mutations (14). A previous study by Cha et al. (23), showed that certain tumor progression patterns, such as rapid progression of primary tumor at progressive disease (PD) were predictive factors for inferior survival in NSCLC patients treated with EGFR-TKIs. On the basis of previous studies, we hypothesized that, since smoking history is related to inferior survival in NSCLC patients, there are differences in radiologic tumor progression pattern between smokers and never-smokers. The aim of our study was to compare survival outcomes after EGFR-TKI therapy according to smoking history and to analyze differences in radiologic tumor progression pattern in order to determine whether smoking history has an impact on survival outcome or radiologic tumor progression pattern in patients with EGFR-mutant NSCLC undergoing EGFR-TKI therapy.
Methods
Our institutional review board (Samsung Medical Center, Seoul, Korea, approval # SMC201403002-HE003) approved this retrospective study with a waiver of informed consent. The patients’ records/information were anonymized and de-identified prior to analysis.
Patients
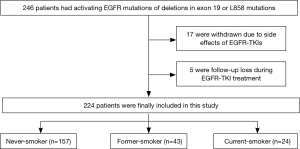
We retrospectively reviewed a total of 246 patients who had histologically proven lung adenocarcinoma in clinical stage IV with confirmed activing EGFR mutations of exon 19 del and exon 21 L858R and were treated with EGFR-TKIs (gefitinib or erlotinib) as first- or second-line therapy, and beyond, at our institution between June 2006 and October 2011. Combined chemotherapy was not performed for any patient during EGFR-TKI therapy. Patients were treated with an EGFR-TKI regimen until either disease progression or the end of the study period. All patients received cycles of EGFR-TKI therapy at three-week intervals and underwent baseline contrast-enhanced chest computed tomography (CT) prior to EGFR-TKI therapy and follow-up contrast-enhanced chest CT after every two EGFR-TKI cycles. We excluded 22 patients from the study because of side effects of EGFR-TKIs before sufficient assessment of treatment response or follow-up loss (Figure 1). As a result, a total of 224 patients were included in the study. We retrospectively reviewed the patients’ medical records for smoking history, categorizing patients as never-smoker (<100 cigarettes in lifetime), former-smoker (quit ≥1 year ago), and current-smoker (smoked at present or quit <1 year ago) (14). We also categorized patients as never-smoker and ever-smoker (former-smokers and current-smokers). Patients were treated with the recommended dose of either erlotinib (150 mg per day, administered orally) or gefitinib (250 mg per day, administered orally) until disease progression was evident.
Imaging and assessment of tumor response
Pretreatment evaluation before EGFR-TKI therapy included chest CT, abdominal CT, bone scan or FDG-positron emission tomography (PET)-CT, and magnetic resonance imaging (MRI) of the brain.
Baseline chest CT scans were performed less than two weeks before EGFR-TKI therapy. Clinical features of the patients and metastasis status at baseline and at PD were collected. Baseline was defined as the state before the start of the EGFR-TKI treatment, and PD was defined as systemic progression or the appearance of new metastatic lesions while on EGFR-TKI treatment, based on the revised Response Evaluation Criteria in Solid Tumors (RECIST) (24). The pattern of metastasis was determined as intra- or extra-thoracic metastasis. Intra-thoracic metastasis was defined as metastasis in contiguous anatomic sites, such as both hemithorax and mediastinum. Extra-thoracic metastasis was defined as metastasis outside the hemithorax and mediastinum. Tumor (T) stage, nodal (N) stage, and metastasis (M) stage were assessed at baseline, as was the number of metastatic sites. At development of PD, we evaluated the patient’s primary tumor and metastatic status with appropriate imaging methods. At PD, primary tumor status was classified as no change, slow progression, or rapid progression. Slow progression was defined as less than 20% increase in the longest diameter of the primary tumor on two follow-up studies and rapid progression was defined as more than 20% increase in the longest diameter of primary tumor on two follow-up studies. Radiologic tumor progression patterns were subgrouped as progression of primary tumor only, intrathoracic metastasis, or extrathoracic metastasis, such as brain, bone, liver, or adrenal gland (23).
Patient follow-up and evaluation of clinical outcomes
All patients underwent planned radiologic assessments at baseline and every six weeks throughout therapy. Treatment response was evaluated by radiologists based on the revised RECIST (24). We examined the disease control rate (DCR), overall response rate (ORR), PFS, and overall survival (OS) of patients on EGFR-TKIs. DCR was defined as the proportion of patients who achieved complete response (CR), partial response (PR), or stable disease (SD) as the best response to EGFR-TKIs. ORR was defined as the proportion of patients with CR or PR. Patients were observed for survival evaluation until death or final hospital visit. PFS was calculated from the start of EGFR-TKIs until disease progression, death without documented progression, or the final follow-up visit. OS was calculated from the start of EGFR-TKIs until death or the final follow-up visit.
Statistical analysis
Differences in clinical variables according to smoking history were tested using the Chi-square test and Fischer’s exact test according to Cochran’s rule. Survival curves were derived using Kaplan-Meier methods. Univariate and multivariate analyses were performed using Cox proportional hazards regression models to identify independent factors associated with survival outcomes. Two-sided P values less than 0.05 were considered significant. All analyses were performed using SPSS version 20.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
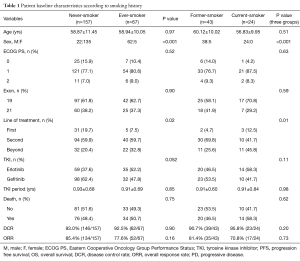
A total of 224 patients with stage IV lung adenocarcinoma with confirmed EGFR mutations were analyzed. Of 224 patients, 157 (70.1%) were never-smokers and 67 (29.9%) were ever-smokers. Of the 67 ever-smokers, 43 were former-smokers and 24 were current-smokers at the time of diagnosis (Figure 1). Patients with former/current smoking history were more likely to have received EGFR-TKIs after other forms of treatment and chemotherapies compared with never-smokers (P=0.02). ORR to EGFR-TKI therapy was 72.2% with first-line, 88.7% with second-line, and 77.8% with third-line and beyond. Of 224 patients, 110 (49%) had died and 202 (90.2%) had PD. Other clinical characteristics such as age, Eastern Cooperative Oncology Group performance status (ECOG PS), and type of EGFR-TKI were not different according to smoking history (Table 1).
Full table
Clinical response of EGFR-TKI and radiologic tumor progression patterns according to smoking history
The DCR and ORR for patients treated with EGFR-TKIs were not different between never-smokers and smokers, but there was a trend of lower DCR and ORR in smokers than never-smokers (DCR, 93.0% vs. 92.5%, ORR, 85.4% vs. 77.6%). The DCR and ORR for EGFR-TKIs among never-, former-, and current-smokers were not significantly different (DCR, 93.0% vs. 90.7% vs. 95.8%, ORR, 85.4% vs. 81.4% vs. 70.8%), but there was a trend of lower ORR in former- and current-smokers than in never-smokers (Table 1).
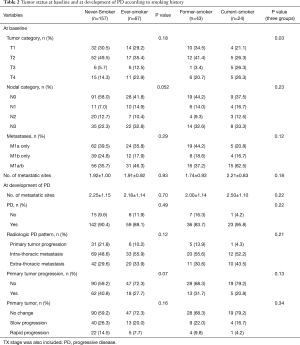
At baseline, T stage was significantly higher in current-smokers than in never- and former-smokers (P=0.03). N and M stages were not significantly different between never-smokers and smokers. Although not significant, current-smokers tended to have more intra- and extra-thoracic metastasis at baseline than never- and former-smokers. At the development of PD, there were no significant differences in radiologic tumor progression pattern or primary tumor status according to smoking history (Table 2).
Full table
Survival outcomes of EGFR-TKI therapy according to smoking history
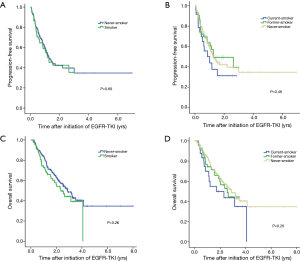
There were no significant differences in survival between never-smokers and smokers (median PFS, 1.25 vs. 1.26 years; median OS, 2.87 vs. 2.35 years) or among never-, former-, and current-smokers (median PFS, 1.25 vs. 1.26 vs. 1.01 years; median OS, 2.87 vs. 2.55 vs. 1.70 years), although there was a trend of shorter PFS and poorer OS in smokers than in never-smokers (Figure 2). In univariate analysis, sex, ECOG PS ≥2, high baseline T stage, M1a or M1b stage, and number of metastatic sites ≥3 were factors for reduced PFS and poor OS in the Cox proportional hazard model. There were no significant differences in any of these factors between never- and long-term-smokers or among never-, former-, and current-smokers (Table 3).
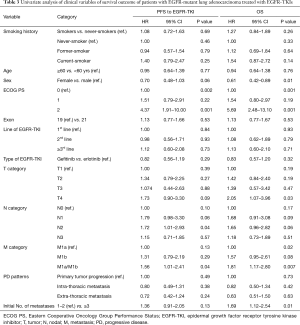
Full table
In multivariate analysis adjusted for age, sex, ECOG PS, EGFR mutation status, line of treatment, type of EGFR-TKI, baseline T stage, N stage, M stage, and number of metastatic sites, smokers had significantly shorter PFS and poorer OS compared to never-smokers (HR, 2.22; 95% CI, 1.10–4.46, P=0.03 for PFS; HR, 2.21; 95% CI, 1.06–4.58, P=0.03 for OS). In addition to smoking history, sex (male sex, P=0.009 for PFS; P=0.02 for OS), ECOG PS ≥2 (P=0.02 for PFS; P=0.001 for OS), and M stage (M1a + M1b, P=0.01 for OS) were found to be associated with reduced PFS and poor OS in the multivariate analysis (Table 4).
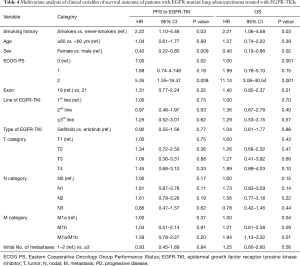
Full table
Discussion
Lung cancer in never-smokers and smokers differs in both clinical features and genomic characteristics (1,2,25). There are several distinct differences in the genomic landscapes of those who have and those who have never smoked. First, significantly higher mutation frequencies are observed in smokers than never-smokers. Second, the spectrum of mutations is different, for example C:G → A:T transversions are predominantly seen in smokers, whereas C:G → T:A transitions are predominantly seen in never-smokers. In addition, distinctive sets of mutations are identified in smokers versus never-smokers (25). These differences in genomic characteristics of never-smokers and smokers might be related to different tumor behaviors, progression patterns, or treatment responses to therapeutic agents such as EGFR-TKIs.
Since there are controversies regarding the survival outcomes of smokers versus non-smokers after EGFR-TKI therapy, we aimed to identify whether there are differences in treatment responses between smokers and non-smokers undergoing EGFR-TKI therapy that are specifically related to EGFR mutant status. Our results showed a trend of inferior survival in smokers; after adjusting for other clinical factors, smoking history was a predictive factor for inferior survival in patients with lung adenocarcinoma. Based on these data, we hypothesize that smoking induces distinct factors that lead to development of tumor progression or acquired resistance to EGFR-TKIs. Cigarette smoke is already known to induce activation of cytochrome CYP 1A1/2, which influences the metabolism of EGFR-TKIs and may reduce the therapeutic response to these drugs (26,27). In the preclinical study by Filosto et al. (21) cigarette smoke stimulated aberrant EGFR phosphorylation that impaired receptor degradation and induced a different EGFR conformation and a signaling pathway that was resistant to EGFR-TKIs. Moreover, other researchers have demonstrated that cigarette smoke induces aberrant phosphorylation of EGFR, resulting in lack of ubiquitination by c-Cbl and impaired degradation. Thus, it is probable that this kind of EGFR activation without the feedback regulation of normal degradation leads to uncontrolled lung cancer growth (22). In another study by Togashi et al. (28), chronic nicotine exposure mediated resistance to EGFR-TKI by inducing EGFR signal activation, and this effect was cancelled by nicotinic acetylcholine receptor inhibitor. Based on these studies, tumors in patients with smoking history probably acquire early resistance to EGFR-TKIs and demonstrate more aggressive tumor behavior than those in never-smokers.
Several studies have shown similar results to those presented here. The latest study by Kim et al. (14) showed that cigarette smoking at a dosage of ≥30 pack-years is an independent negative predictive factor of EGFR-TKI treatment outcome in patients with lung adenocarcinoma with activating EGFR mutations. They showed significant differences in PFS and OS according to smoking history in univariate analysis. In the study by Fukuhara et al. (29), smokers had significantly lower ORR than never-smokers, and patients with the exon 21 L858R mutation had a poorer response to gefitinib treatment than those with exon 19 del. However, in our study, EGFR mutation status had no significant effect on the clinical outcome. Also, recent meta-analyses (17,30) showed that non-smokers are likely to have a longer PFS than smokers when undergoing EGFR-TKI therapy for diagnosed EGFR-mutant NSCLC. A recent study by Mitchell et al. (16) concluded that controversies in the survival outcomes of smokers versus non-smokers after EGFR-TKI therapy may be due to incomplete data on smoking history, and that its relationship with treatment response has not been comprehensively analyzed.
In our study and the study by Kim et al. (14), patients with smoking history were more likely to have received other treatments or chemotherapies before EGFR-TKI therapy than were never-smokers. This factor did not reach statistical significance in univariate or multivariate analysis. In previous studies (31,32), survival was not different between patients receiving first- or second-line EGFR-TKI treatment, demonstrating the comparable efficacy of EGFR-TKIs as second-line treatment after failure of chemotherapy.
There is one available study on favorable CT features in patients with EGFR mutations (33). However, there is no information about radiologic tumor progression patterns based on smoking history, and we believe that our study is the first to analyze radiologic tumor progression patterns according to smoking history. Based on previous preclinical studies (21,22), we investigated the difference in progression patterns between patients who have and have not smoked. However, our study showed no significant differences in radiologic tumor progression pattern, including primary tumor status, based on smoking history. Since smokers with lung cancer have higher numbers of genomic alterations than never-smokers with lung cancer, it is believed that the vast majority of these genomic alterations do not have any role in malignant transformation or progression of tumor growth or metastasis (34). This might explain why our results showed no significant differences in tumor progression patterns according to smoking history. However, our study showed a trend of increased primary tumor progression in never-smokers than smokers and more intra-or extrathoracic metastasis in the smokers than never-smokers. Supporting our results, a study by Huynh et al. (35) showed that cigarette smoke induces changes in claudin expression, which may be important in lung cancer biology as alterations in claudin can cause tumor invasiveness, decreased cell adhesion, uncontrolled cell proliferation, loss of differentiation, and loss of cohesion, all of which contribute to lung cancer progression and possibly contribute to metastasis. As EGFR amplification is associated with tumor invasiveness and metastasis (36), it might also have some relationship with smoking. It is known that cigarette smoke causes damage in DNA, including deletions in microRNA-encoding genes, leading to lung cancer and uncontrolled cellular growth (37). Further studies are needed to validate these correlations.
Our study had several limitations. This study was performed retrospectively with limited sample sizes. Since smoking history was assessed at baseline, smoking status during treatment was not monitored. This is important because the smoking habits of patients with NSCLC can fluctuate and might affect the treatment response (38). Furthermore, exposure to second-hand smoke was not assessed. The impact of second-hand smoke might play an important role in etiology of NSCLC in those who have never smoked (39). We also did not assess other environmental lung carcinogens, such as airborne pollution. There was also an imbalance in baseline proportions of sex and line of EGFR-TKIs between never-smokers and smokers, which might have caused discrepancy in the variable of smoking history between univariate and multivariate analyses. Imbalance of baseline proportions of sex between never-smokers and smokers is probably due to the high number of female Asian patients with lung adenocarcinomas included in our study. In addition, we only included activating EGFR mutations of exon 19 del and exon 21 L858R in our study design. Since lung cancer is not a single hit but requires multiple mutations, it is possible that other mutations play a role in determining the influence of smoking on clinical outcome.
In conclusion, this study demonstrated that smoking history has no significant influence on radiologic tumor progression pattern, but is predictive of inferior survival in patients with EGFR-mutant lung adenocarcinoma undergoing EGFR-TKI therapy. Therefore, smoking history should be considered as part of the prognosis in studies on EGFR-TKIs as treatment for EGFR mutant lung adenocarcinoma. Additional prospective studies should be carried out to validate these results.
Acknowledgements
Funding: This study was supported by a grant from the Korean Foundation for Cancer Research (KFCR-CB-2011-02-02).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our institutional review board (Samsung Medical Center, Seoul, Korea, approval # SMC201403002-HE003) approved this retrospective study with a waiver of informed consent. The patients’ records/information were anonymized and de-identified prior to analysis.
References
- Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer 2007;7:778-90. [Crossref] [PubMed]
- Lee YJ, Kim JH, Kim SK, et al. Lung cancer in never smokers: change of a mindset in the molecular era. Lung Cancer 2011;72:9-15. [Crossref] [PubMed]
- Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer 2006;118:257-62. [Crossref] [PubMed]
- Matsuo K, Ito H, Yatabe Y, et al. Risk factors differ for non-small-cell lung cancers with and without EGFR mutation: assessment of smoking and sex by a case-control study in Japanese. Cancer Sci 2007;98:96-101. [Crossref] [PubMed]
- Hsieh RK, Lim KH, Kuo HT, et al. Female sex and bronchioloalveolar pathologic subtype predict EGFR mutations in non-small cell lung cancer. Chest 2005;128:317-21. [Crossref] [PubMed]
- Shepherd FA, Rodrigues Pereira J, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2005;23:5892-9. [Crossref] [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [Crossref] [PubMed]
- Ahn MJ, Park BB, Ahn JS, et al. Are there any ethnic differences in molecular predictors of erlotinib efficacy in advanced non-small cell lung cancer? Clin Cancer Res 2008;14:3860-6. [Crossref] [PubMed]
- Tomizawa Y, Iijima H, Sunaga N, et al. Clinicopathologic significance of the mutations of the epidermal growth factor receptor gene in patients with non-small cell lung cancer. Clin Cancer Res 2005;11:6816-22. [Crossref] [PubMed]
- Li AR, Chitale D, Riely GJ, et al. EGFR mutations in lung adenocarcinomas: clinical testing experience and relationship to EGFR gene copy number and immunohistochemical expression. J Mol Diagn 2008;10:242-8. [Crossref] [PubMed]
- D'Angelo SP, Pietanza MC, Johnson ML, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol 2011;29:2066-70. [Crossref] [PubMed]
- Linardou H, Dahabreh IJ, Bafaloukos D, et al. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat Rev Clin Oncol 2009;6:352-66. [Crossref] [PubMed]
- Kim MH, Kim HR, Cho BC, et al. Impact of cigarette smoking on response to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors in lung adenocarcinoma with activating EGFR mutations. Lung Cancer 2014;84:196-202. [Crossref] [PubMed]
- Banno E, Togashi Y, Kobayashi Y, et al. Afatinib is especially effective against non-small cell lung cancer carrying an EGFR exon 19 deletion. Anticancer Res 2015;35:2005-8. [PubMed]
- Mitchell P, Mok T, Barraclough H, et al. Smoking history as a predictive factor of treatment response in advanced non-small-cell lung cancer: a systematic review. Clin Lung Cancer 2012;13:239-51. [Crossref] [PubMed]
- Zhang Y, Kang S, Fang W, et al. Impact of smoking status on EGFR-TKI efficacy for advanced non-small-cell lung cancer in EGFR mutants: a meta-analysis. Clin Lung Cancer 2015;16:144-151.e1. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Filosto S, Becker CR, Goldkorn T. Cigarette smoke induces aberrant EGF receptor activation that mediates lung cancer development and resistance to tyrosine kinase inhibitors. Mol Cancer Ther 2012;11:795-804. [Crossref] [PubMed]
- Khan EM, Lanir R, Danielson AR, et al. Epidermal growth factor receptor exposed to cigarette smoke is aberrantly activated and undergoes perinuclear trafficking. FASEB J 2008;22:910-7. [Crossref] [PubMed]
- Cha YK, Lee HY, Ahn MJ, et al. Survival outcome assessed according to tumor burden and progression patterns in patients with epidermal growth factor receptor mutant lung adenocarcinoma undergoing epidermal growth factor receptor tyrosine kinase inhibitor therapy. Clin Lung Cancer 2015;16:228-36. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012;150:1121-34. [Crossref] [PubMed]
- Mir O, Blanchet B, Goldwasser F. Drug-induced effects on erlotinib metabolism. N Engl J Med 2011;365:379-80. [Crossref] [PubMed]
- Cortot AB, Jänne PA. Molecular mechanisms of resistance in epidermal growth factor receptor-mutant lung adenocarcinomas. Eur Respir Rev 2014;23:356-66. [Crossref] [PubMed]
- Togashi Y, Hayashi H, Okamoto K, et al. Chronic nicotine exposure mediates resistance to EGFR-TKI in EGFR-mutated lung cancer via an EGFR signal. Lung Cancer 2015;88:16-23. [Crossref] [PubMed]
- Fukuhara T, Maemondo M, Inoue A, et al. Factors associated with a poor response to gefitinib in the NEJ002 study: smoking and the L858R mutation. Lung Cancer 2015;88:181-6. [Crossref] [PubMed]
- Hasegawa Y, Ando M, Maemondo M, et al. The role of smoking status on the progression-free survival of non-small cell lung cancer patients harboring activating epidermal growth factor receptor (EGFR) mutations receiving first-line EGFR tyrosine kinase inhibitor versus platinum doublet chemotherapy: a meta-analysis of prospective randomized trials. Oncologist 2015;20:307-15. [Crossref] [PubMed]
- Lee JK, Shin JY, Kim S, et al. Primary resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with non-small-cell lung cancer harboring TKI-sensitive EGFR mutations: an exploratory study. Ann Oncol 2013;24:2080-7. [Crossref] [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [Crossref] [PubMed]
- Choi CM, Kim MY, Lee JC, et al. Advanced lung adenocarcinoma harboring a mutation of the epidermal growth factor receptor: CT findings after tyrosine kinase inhibitor therapy. Radiology 2014;270:574-82. [Crossref] [PubMed]
- Subramanian J, Govindan R. Molecular profile of lung cancer in never smokers. EJC Suppl 2013;11:248-53. [Crossref] [PubMed]
- Huynh TP, Mah V, Sampson VB, et al. Na,K-ATPase is a target of cigarette smoke and reduced expression predicts poor patient outcome of smokers with lung cancer. Am J Physiol Lung Cell Mol Physiol 2012;302:L1150-8. [Crossref] [PubMed]
- Jakobsen JN, Sørensen JB. Intratumor heterogeneity and chemotherapy-induced changes in EGFR status in non-small cell lung cancer. Cancer Chemother Pharmacol 2012;69:289-99. [Crossref] [PubMed]
- Izzotti A, Pulliero A. Molecular damage and lung tumors in cigarette smoke-exposed mice. Ann N Y Acad Sci 2015;1340:75-83. [Crossref] [PubMed]
- Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev 2005;14:2287-93. [Crossref] [PubMed]
- Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol 2007;25:561-70. [Crossref] [PubMed]


