|
Short Communication
The sleep apnea cardiovascular endpoints (SAVE) trial: Rationale and start-up phase
R Doug McEvoy 1, Craig S Anderson 2, Nick A Antic 1, Baoyuan Chen 3, Quanying He 4, Emma Heeley 2, Shaoguang Huang 5, Yining Huang 6, Jiguang Wang 7, Nanshan Zhong 8
1The Adelaide Institute for Sleep Health, Repatriation General Hospital, Daw Park, SA, 5000. Australia; 2The George Institute for Global Health, Royal Prince Alfred Hospital and University of Sdyney, Camperdown, NSW 2050, Australia; 3Respiratory Medicine, General Hospital of Tianjin Medical University, Tianjin, China; 4Respiratory Medicine, Beijing University People's Hospital, Beijing, China; 5Respiratory Medicine, Ruijin Hospital, Shanghai, China; 6Neurology, Peking First University Hospital, Beijing, China; 7Shanghai Institute of Hypertension, Ruijin Hospital, Shanghai, China; 8Respiratory Medicine, The First Affiliated Hospital of Guangzhou Medical College
Corresponding Author: R Doug McEvoy, MD. The Adelaide Institute for Sleep Health, Repatriation General Hospital, Daw Park, SA, 5000, Australia. Phone: +61 8 8275 1187; Fax: +61 8 8277 6890. Email: doug.mcevoy@health.sa.gov.au
|
|
Abstract
The sleep apnea cardiovascular endpoints (SAVE) study (Clinical Trials Registration Number: NCT00738170) is
an academic initiated and conducted, multinational, open, blinded endpoint, randomised controlled trial designed
to determine whether treatment of obstructive sleep apnea (OSA) with continuous positive airways pressure (CPAP)
can reduce the incidence of serious cardiovascular events in patients with established cardiovascular disease. The
answer to this question is of major importance to populations undergoing ageing and lifestyle changes all over the
world. The SAVE study brings together respiratory, sleep and cardiovascular clinician-scientists in a unique interdisciplinary
collaborative effort with industry sponsors to conduct the largest and most ambitious clinical trial yet
conducted in the field of sleep apnea, with a global recruitment target of 5000 patients. Following its launch in Australia
and China in late 2008, SAVE has now entered a phase of international expansion with new recruitment networks
being established in New Zealand, India and Latin America. This article describes the rationale for the SAVE
study, the considerations behind its design, and progress thus far in establishing the recruitment network. The report
emphasises the important role that Chinese sleep and cardiovascular investigators have played in the start-up phase
of this landmark international project.
Key words
obstructive sleep apnea; continuous positive airways pressure; sleep apnea cardiovascular endpoints (SAVE) study
J Thorac Dis 2010;2:138-143. DOI: 10.3978/j.issn.2072-1439.2010.02.03.5
|
|
Rationale for the SAVE study
Obstructive sleep apnea (OSA) was first widely recognised as a
clinical disorder in the 1970s. OSA is characterised by episodic,
complete or partial upper airway obstruction during sleep which
leads to sleep fragmentation and intermittent hypoxaemia,
tachycardia and surges in systemic and pulmonary arterial blood
pressure. In 1981, nasal continuous positive airway pressure
(CPAP) was shown to be a highly effective, low-risk treatment for
OSA patients ( 1). The major focus of treatment until now has been
to relieve patients of debilitating daytime sleepiness and socially disruptive snoring. However, over the last two decades, there has
been increasing evidence of a possible causal relationship between
OSA and cardiovascular disease. A number of pathways have been
proposed by which night-time physiological disturbances in OSA
patients might lead to cardiovascular disease or cardiovascular
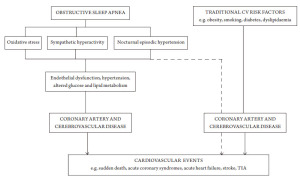
events ( Fig 1). Experiments in animals exposed to patterns of intermittent
hypoxia similar to those experienced by patients with OSA
have shown sustained elevations in blood pressure, central
nervous system damage, and abnormalities of glucose and
lipid metabolism ( 2-5). Clinical observational and casecontrol
studies in patients have shown that OSA appears
to be independently associated with hypertension, glucose
dysregulation and ischaemic heart disease and cerebrovascular
disease ( 6-10). These observations have been supported by
the results of several large population surveys which have
also shown a possible link between OSA and cardiovascular
morbidity ( 11-14) and mortality ( 15-17). Short-term CPAP
treatment intervention studies in OSA patients have shown small reductions in systemic ( 18, 19) and pulmonary artery
pressure ( 20, 21), and improvements in some other biomarkers
of cardiovascular risk ( 22), although not all such treatment
intervention studies have been positive ( 23). Thus, the evidence has clearly been mounting that OSA may
increase the risk of premature cardiovascular disease including
myocardial infarction and stroke, and that treatment of OSA
may reduce these risks. However, the ultimate test of whether
a pathophysiological disorder such as OSA causes premature
cardiovascular disease and whether treatment of the disorder
reduces cardiovascular risk, requires long-term, large-scale,
randomised controlled trials that compare the incidence of
“hard” cardiovascular outcomes in patients who are treated for
OSA and those who are not. This point has been emphasised
in recent leading editorials and scientific consensus statements
on the topic ( 24, 25). For example, the 2008 joint American
Heart Association and American College of Cardiologists
Foundation Scientific statement on sleep apnea ( 25) concluded
that the observational nature of most of the evidence and the
possibility of residual confounding by visceral obesity weakened
the overall case in favour of a causal link between OSA and
cardiovascular disease. This statement concluded that longterm
properly designed intervention studies showing benefit
from OSA treatment are missing and this lack of evidence is
limiting progress in this field. The SAVE trial (Clinical Trials
Registration Number: NCT00738170) has been designed to
help fill this evidence gap.
|
|
Background planning, study design
and trial management
Planning for the SAVE trial began in 2006. An academic
partnership was formed between Australian sleep and
respiratory clinician scientists at the Adelaide Institute for
Sleep Health (AISH), Flinders University, and cardiovascular
clinical epidemiologists at the George Institute of Global
Health at the University of Sydney, to prepare the ground
work for the trial. The George Institute, which has previously
conducted a number of large-scale international clinical trials
in cardiovascular medicine (e.g. PROGRESS, ONTARGET/
TRANSCEND, ADVANCE, INTERACT), provided the
necessary expertise and research infrastructure to mount a trial
of this size. The AISH had considerable experience in clinical
sleep research and, with the assistance of the Australasian
Sleep Trials Network, provides the necessary expertise in the
areas of sleep apnea diagnosis and CPAP treatment. An untied
priming grant was provided by the Respironics Foundation
(USA) in late 2005, to begin a feasibility and scoping exercise to determine the best way to proceed in the conduct of SAVE.
With these funds, extensive discussions were conducted
internationally amongst sleep, respiratory and cardiovascular
investigators and, with additional equipment grants from the
device companies Compumedics and ResMed, a preliminary
study was conducted in Shanghai to test the validity of a simple
screening device for diagnosing OSA in patients entering the
trial. Work then began on the final study design and research
plan, and a major untied start-up grant was provide by the
Respironics Foundation to develop and launch the study.
It was decided that the study would be a randomised
control trial of CPAP treatment in OSA patients focussing on
“hard” cardiovascular endpoints such as myocardial infarction
and stroke, rather than confined to surrogate markers of
cardiovascular risk such as blood pressure, lipids and glucose
metabolism. The rationale for this decision was twofold. First,
we believed that the results of such a trial would ultimately have
a much greater impact on clinical decision making than another
trial focussing on known secondary markers of cardiovascular
risk. Secondly, the pathogenic pathways whereby OSA could
lead to CV events are likely to be multiple and the relative
importance of one mechanism over another is unknown. Thus,
we considered an approach that measured the outcome of
CPAP treatment on a composite of downstream cardiovascular
events, and which did not assume a dominant mechanism or
mechanisms for increased cardiovascular risk, was preferable.
Having made this decision, there were four important remaining
questions. (i)Would the study be best designed as a primary
or secondary prevention RCT? (ii) How and where would the
patients be recruited? (iii) Should the diagnosis of OSA be
made using an ambulatory screening device or would it require
hospital sleep laboratory facilities? (iv) What were the important
ethical considerations in randomising approximately half the
OSA patients to no CPAP treatment for several years? and (v)
How would reasonable levels of adherence to CPAP treatment
be guaranteed, recognising that most of the patients enrolled
in this the trial were likely to be ‘minimally sleepy’ or ‘not at all
sleepy’, and therefore, may perceive relatively little symptomatic
benefit from the treatment? Also, each patient would need to be
followed for several years.
Primary versus secondary prevention trial
design
Because of the relatively high cardiovascular event rates
amongst patients who have established cardiovascular
disease, the numbers of patients that need to be enrolled in a
secondary prevention trial are generally in the order of 20-30%
of the number needed for a primary prevention trial. While
a secondary prevention trial design may target somewhat
different pathogenic mechanisms for cardiovascular disease
than a primary prevention study ( Fig 1), it nevertheless has the potential to provide highly relevant information with which to
inform clinical practice and reduce cardiovascular morbidity
and mortality. Furthermore, it increases the overall feasibility
of successfully completing such a trial, given the smaller
number of patients and shorter period of follow-up required,
and thus, ultimately, lower overall trial cost.
Patient recruitment – where and how?
A secondary prevention trial of OSA treatment requires access
to a large number of patients with co-existing cardiovascular
disease and OSA who are willing to be entered with a 50:50
chance of being allocated to active OSA treatment or usual
care control. It was considered that this would not be easily
achieved by relying solely on patients referred to sleep
medicine services. As well as the numbers of patients referred
to sleep services being lower in comparison to the numbers
referred to cardiovascular clinics, it is likely that the proportion
of sleep clinic patients with co-existing cardiovascular disease
is likely to be relatively low (ie in the order of 10% ( 6)). Even
more important, however, is that sleep clinic patients will,
in the main, be symptomatic and expect to be offered OSA
treatment. The alternative method of recruiting patients
directly from cardiovascular clinics is more attractive,
although it has its own challenges. It is known that the
prevalence of OSA is about 30 to 60% in high cardiovascular
risk populations ( 25-29). Thus, cardiovascular clinics are
potentially a rich source of patients for SAVE. However, we
were aware that this would either require cardiovascular
clinician researchers to develop new skills in sleep apnea
diagnosis and CPAP treatment or for respiratory/ sleep and
cardiovascular physicians to form strong collaborative working
relationships at the local site level. In the end, it was decided
to invite both sleep/ respiratory and cardiovascular clinicians
to participate in the study, to provide training and support
in sleep diagnostics and CPAP therapy where needed, and to
encourage collaboration between the medical disciplines at
national and local hospital levels.
Diagnosis of OSA - ambulator y sleep
apnea moni tor ver sus in- laborator y
polysomnography (PSG)
It has become apparent to experts in the sleep field ( 30)
that in-laboratory polysomnography (PSG) is not a costeffective
method to screen and diagnose OSA large numbers
of subject that are required for a study such as SAVE. As well
as it being a high-cost and labour intensive test to perform,
there is considerable cost and effort required to standardise
PSG recording and scoring techniques between laboratories
(or centralise scoring), which in a study with multiple sites,
was considered prohibitive. Furthermore, we were aware that
many of our potential recruitment sites in China did not have access to PSG and many Australian sites with PSG facilities
were already heavily booked with clinical work. There has been
increasing evidence that simplified home screening devices
can be used to identify cases of at least moderate-severe OSA
with a high degree of certainty, at least in populations with
a high pre-test probability of disease. Since the prevalence
of OSA in the proposed study population (i.e. patients with
established coronary- or cerebro-vascular disease) was likely
to be high, it was decided to validate a simple, automated
2-channel (oximetry and nasal pressure) screening device
(the ApneaLink, ResMed, Sydney, Australia) in a high
cardiovascular risk population in China with the view to using
this device in the main study. Chinese and Australian clinician
scientists collaborated on this validation study which was
completed in Shanghai in early 2008. It was found in 143 high
cardiovascular risk patients that the automatically calculated
oxygen desaturation (oximetry) and apnea-hypopnea (nasal
pressure) indices had equally high diagnostic accuracy
for moderate-severe OSA when compared with full PSG
simultaneously performed in the patients’ homes (Gantner
et al Respirology in press). ApneaLink Oximetry had a lower
technical failure rate (e.g. loss of signal because of sensor
displacement) than nasal pressure recordings making oximetry
the preferred primary diagnostic method for identifying OSA
patients for SAVE. The nasal pressure trace is used to exclude
patients whose predominant pattern of sleep disordered
breathing pattern is symmetrical waxing and waning of flow
indicating Cheyne-Stokes respiration.
Ethical issues
The main ethical issue relevant to the SAVE study relates to
the withholding of CPAP treatment in approximately half the
patients who screen positive for OSA, when they may stand to
benefit from reduction in daytime sleepiness, and improved
driving safety and quality of life with therapy. Since patients
in SAVE are recruited from cardiovascular clinics, almost
all participants will be unaware of having had OSA prior to
the SAVE screening diagnostic evaluations. While previous
studies have shown that the great majority of patients with
co-existing cardiovascular disease and OSA have little to no
daytime symptoms, to minimise any safety concerns it was
decided to exclude patients who held a commercial drivers,
who demonstrated marked daytime sleepiness (defined as
Epworth Sleepiness Scale score > 15), or who reported a fallasleep
accident or near miss accident in the 12 months prior
to enrolment. An independent Data Safety Monitoring Board
(DSMB) was established to monitor the rates of self-reported
accidents in the CPAP-treated and non CPAP-treated groups
at regular intervals during the course of the study. At the time
of enrolment, all patients are given a full explanation of the
possible symptomatic benefits of CPAP and given the option to seek treatment outside the trial if they wish, and both the
patient and their responsible physician must be comfortable
about them being randomly allocated to CPAP treatment or no
CPAP treatment.
Long term CPAP adherence
It was considered that adherence to CPAP therapy might
be a significant problem in a long-term study of several
years duration, particularly considering that the majority of
subjects were likely to report little or no daytime sleepiness
and therefore would be unlikely to experience significant
symptomatic benefit. In addition it is known from clinical
studies that as many as 30% of sleep apnea patients refuse
CPAP treatment outright or in the first few weeks of therapy
( 31). To exclude those patients who were unwilling or unlikely
to adhere to CPAP therapy, we decided to use a one-week sham
CPAP run-in phase. Sham CPAP is designed to deliver only a
very low, non-therapeutic pressure to the airway, and previous
clinic studies have shown similar short-term adherence levels
with sham and active CPAP. We argued that if patients were
sufficiently motivated and able to wear a CPAP mask for a
minimum average period of 3 hours per night, they would
likely comply long-term with active treatment during the trial.
Trial management
The trial is led by an Executive Committee who has overall
responsibility for the design and proper conduct of the
study. Day-to-day operational matters are decided by an
Operations Committee. A Principal Investigator (PI) for
each participating country or region advises the Executive
and Operations Committees on relevant national regulatory
issues, clinical practice standards, and patient recruitment
strategies. In China, the national PI is assisted by a China PI
committee consisting of a small group of experts in sleep and
respiratory medicine and stroke and cardiovascular medicine.
A DSMB has regular oversight of the study and is responsible
for safeguarding the interests of trial participants, assessing
the safety and efficacy of the interventions during the trial,
and for monitoring the overall conduct of the trial. The DSMB
will undertake 2 planned interim analyses and will provide
recommendations about stopping or continuing the trial to
the trial Executive Committee. Further information regarding
the structure and composition of the various SAVE trial
committees is provided in Appendix A.
|
|
Progress during the start-up phase
In China, a total of 40 sites were sequentially initiated over
the period from November 2008 to May 2010, and have
successfully recruited patients. A total of 15 sites were
established in Australia and New Zealand over the same period. Start-up meetings were conducted in China and
Australia in June and October 2008, respectively, at which
training in sleep diagnostics, CPAP treatment and patient
recruitment and good clinical trial practice was undertaken.
This has been reinforced by one-on-one training of site
coordinators by SAVE trial clinical research associates at the
local hospital level.
As of 2nd July 2010, 590 patients had been randomised in the
trial, 477 in China, 102 in Australia and 11 in New Zealand.
The average rate of patients recruited per month per active site
has varied from about 0.8 to 1.0, with a very large variation
in recruitment efficiency between sites. These recruitment
rates are lower than initially envisaged and major efforts are
currently underway to lift the recruitment rate at individual
sites wherever possible, and to expand the recruitment network
to include India, Latin America and the United Kingdom.
The impediments to recruitment have varied from time
to time, between sites and between countries. The major
difficulty experienced by investigators with recruitment
relates to the relative complexity and time consuming nature
of the screening process. A decision was made in early 2009
to reduce the amount of unnecessary data collection and data
entry for investigators. Notwithstanding these changes, the
study requires a fairly high level of technical competence and
intervention (e.g. sleep apnea diagnosis, sham CPAP run-in,
and therapeutic CPAP implementation) that is unavoidable.
Access to a suitable high cardiovascular risk patient pool is a
problem in some sites but not others. Generally, recruitment
rates are highest at sites which have good access to high
cardiovascular risk patients and have a highly motivated and
well organised study coordinator who has sufficient time to
devote to the trial work. Early trends show that average CPAP
adherence rates are in the range of 4.5 to 5 hours per night at 6
months, which compares favourably with previously reported
CPAP adherence rates in symptomatic clinic population ( 31).
This excellent result is likely due to a combination of factors,
including: use of a sham run-in phase to exclude subjects
unable to tolerate mask treatment; a study population which
by virtue of the serious nature of their cardiovascular problems
is motivated to comply with the study procedures; and the
intensive training and support in CPAP therapy that has been
provided to site investigators, which has led to a high level
of proficiency in CPAP despite, in many cases, little or no
previous investigator experience in this field.
|
|
Summary and conclusions
The SAVE trial is by far the largest and most ambitious clinical
trial yet conceived in the field of sleep apnea research. The
trial has been made possible by the early generous support of
industry. However, SAVE was independently conceived and designed by academics in sleep and cardiovascular medicine
who have complete autonomy with respect to the conduct of
the study and analysis of trial data. The SAVE trial is in its early
phase but has already attracted considerable international
interest and support from leaders in sleep, respiratory and
cardiovascular medicine, with over 600 patients enrolled to
date. If the results prove that the treatment of OSA reduces
the risk of future serious adverse cardiovascular events in
high cardiovascular risk patients, it will open the door to a
significant new form of therapy for cardiovascular disease risk
reduction, which will have global reach. However, a concerted
and sustained international effort will be required for the
trial to reach a successful conclusion and ultimately, this will
hinge on the dedication and enthusiasm of the individual
investigators and participating centres to recruit and follow-up
sufficient numbers of high-risk patients. Chinese investigators
have played a pivotal early role in the study and China will
soon have enrolled 500 patients into the study. It is hoped that
as the international network expands China will continue to
play a major role in the SAVE trial.
|
|
Acknowledgements
Philips-Respironics is the current major sponsor for the SAVE
study. Philips-Respironics has donated all CPAP equipment
for the trial. ResMed has donated home sleep apnea diagnostic
screening devices. Fisher and Paykel, and the Australasian
Sleep Trials Network have provided additional monetary
support. SAVE is supported by the Adelaide Institute for Sleep
Health and the George Institute for Global Health. The SAVE
PI and Co-PI are supported by research fellowships from the
Australian National Health and Medical Research Council
(NHMRC).
|
|
APPENDIX A
SAVE Executive Committee
RD McEvoy (PI), CA Anderson (co-PI) , RR Grunstein, B
Neal, S Redline, L Palmer, SG Huang, NS Zhong, JG Wang, J
Hedner, G Lorenzi-Filho, N Ramakrishnan.
SAVE Operations Committee
E Heeley (Chair), RD McEvoy, CA Anderson, Antic NA
Data Safety Monitoring Board
GG Jennings (Chair), L Wong , G Marks , S Heritier
(statistician)
China PI Committee
NS Zhong (Chair), BY Chen, QY He, SG Haung, JG Wang, H
Yining
|
|
References
- Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure
applied through the nares. Lancet 1981;1:862-65.[LinkOut]
- Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, et al. Intermittent hypoxia induces hyperlipidemia in lean mice.
Circ Res 2005;97:698-706. [LinkOut]
- Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O’Doherty RM, et al. Intermittent hypoxia causes insulin resistance
in lean mice independent of autonomic activity. Am J Respir Crit Care Med 2007;175:851-7. [LinkOut]
- Fletcher EC, Lesske J, Qian W, Miller CC 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure
in rats. Hypertension 1992;19:555-61. [LinkOut]
- Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence
with oxidative injury to sleep-wake brain regions. Sleep 2004;27:194-201. [LinkOut]
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea
with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [LinkOut]
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin
V. Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. N Engl J Med 2005;353:2034-41. [LinkOut]
- Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc
Am Thorac Soc 2008;5:207-17. [LinkOut]
- McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched
controlled study. Am J Respir Crit Care Med 2007;175:190-5. [LinkOut]
- He QY, Feng J, Zhang XL, Liang ZA, Huang SG, Kang J, et al. Relationship of daytime blood pressure and severity of obstructive
sleep apnea among Chinese: a multi-center investigation in China. Chin Med J (Engl) 2010;123:18-22. [LinkOut]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension.
N Engl J Med 2000;342:1378-84. [LinkOut]
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, et al. Sleep-disordered breathing and cardiovascular disease:
cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19-25. [LinkOut]
- Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, et al. Population-based study of sleep-disordered breathing as a risk
factor for hypertension. Arch.Intern.Med 1997;157:1746-52. [LinkOut]
- Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident
stroke: the sleep heart health study. Am J Respir Crit Care Med 2010;182:269-77. [LinkOut]
- Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause
mortality: the Busselton Health Study. Sleep 2008;31:1079-85. [LinkOut]
- Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a
prospective cohort study. PLoS Med 2009;6:e1000132. [LinkOut]
- Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008;31:1071-8.[LinkOut]
- Barbé F, Durán-Cantolla J, Capote F, de la Peña M, Chiner E, Masa JF, et al. Long-term effect of continuous positive airway
pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med 2010;181:718-26. [LinkOut]
- Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive
sleep apnea. Hypertension 2007;50:417-23. [LinkOut]
- Arias MA, Garcia-Rio F, Alonso-Fernandez A, Martinez I, Villamor
J. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled
crossover study. Eur Heart J 2006;27:1106-13. [LinkOut]
- Sajkov D, Wang T, Saunders NA, Bune AJ, Mcevoy RD. Continuous positive airway pressure treatment improves pulmonary hemodynamics
in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2002;165:152-8. [LinkOut]
- Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs
of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 2007;176:706-12. [LinkOut]
- Kohler M, Ayers L, Pepperell JC, Packwood KL, Ferry B, Crosthwaite N, et al. Effects of continuous positive airway pressure
on systemic inflammation in patients with moderate to severe obstructive sleep apnoea: a randomised controlled trial. Thorax
2009;64:67-73. [LinkOut]
- Somers VK. Sleep --A New Cardiovascular Frontier. N Engl J Med 2005;353:2070-3. [LinkOut]
- Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart
Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for
High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council
on Cardiovascular Nursing. J Am Coll Cardiol 2008;52:686-717. [LinkOut]
- Peker Y, Kraiczi H, Hedner J, Loth S, Johansson A, Bende M. An independent association between obstructive sleep apnoea and
coronary artery disease. Eur Respir J 1999;14:179-84. [LinkOut]
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in women: occurrence and association with
coronary artery disease. Am J Med 1996;101:251-6. [LinkOut]
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest
1996;109:659-63. [LinkOut]
- Lee CH, Khoo SM, Tai BC, Chong EY, Lau C, Than Y, et al. Obstructive sleep apnea in patients admitted for acute myocardial
infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest 2009;135:1488-95. [LinkOut]
- Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc
Dis 2009;51:434-51. [LinkOut]
- Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc
Am Thorac Soc 2008;5:173-8. [LinkOut]
Cite this article as: McEvoy RD, Anderson CS, Antic NA, Chen BY, He QY, Heeley E, Huang SG, Huang YN, Wang JG, Zhong NS. The sleep apnea cardiovascular endpoints (SAVE) trial: Rationale and start-up phase. J Thorac Dis 2010;2(3):138-143. doi: 10.3978/j.issn.2072-1439.2010.02.03.5
|