Percutaneous epicardial ablation of incessant atrial tachycardia originating from the left atrial appendage
Introduction
Several studies have reported ablations for the focal atrial tachycardia (AT) in the left atrial appendage (LAA) (1,2). Rarely, epicardial LAA tachycardia has been reported (3,4). Ablation for epicardial LAA tachycardia can be difficult to carry despite the use of three-dimensional (3D) electroanatomical mapping system. Here, we describe a successfully ablated AT arising from the epicardial LAA area suggestive of ligament of Marshall (LOM) muscle sleeve as regarding the epicardial sharp potentials under guidance of a circular mapping catheter in a patient with structurally normal heart after failed attempts at conventional endocardial ablation.
Case presentation
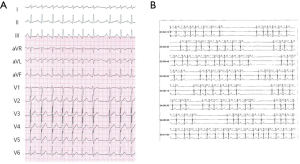
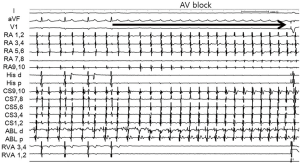
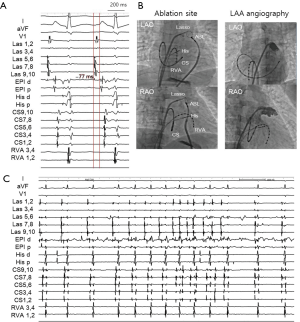
A 38-year-old woman with incessant AT refractory to multiple antiarrhythmic drugs was referred for catheter ablation. On a 12-lead electrocardiogram recorded during AT, the P waves were positive in V1 and inferior leads but negative in lead I and aVL (Figure 1A). Holter recording revealed incessant episodes of AT with a rate of 173/min followed by a long sinus pause (Figure 1B). Echocardiography demonstrated no structural abnormalities. The baseline of electrophysiologic study revealed that the incessant AT, which demonstrated the earliest atrial activation at the distal coronary sinus (CS), was intermittent. At the neck of the LAA, the local electrogram preceded the distal CS signal by 80 ms. After LAA angiography via a transseptal approach, a circular mapping catheter (LASSO 2515 Variable Circular Mapping Catheter, Biosense Webster, Diamond Bar, CA, USA) was placed within the LAA. As guided by circular mapping catheter within LAA, multiple radiofrequency (RF) applications with a standard 4-mm ablation catheter (ABL) only transiently suppressed the atrioventricular (AV) node but did not terminate the AT (Figure 2). After that, epicardial access was performed by pericardial puncture from a percutaneous subxiphoid approach. Under guidance of the circular mapping catheter, careful mapping at the epicardial surface to the neck of the LAA at the opposite site to the endocardial earliest activation site revealed the earliest activation relative to P wave onset (−77 ms, Figure 3A,B). Prior to epicardial ablation as well as endocardial ablation, we performed the high output pacing to avoid phrenic nerve stimulation.
RF energy, using an irrigated-tip ABL, was applied at the epicardial site (30 W, 42 °C, for 60 seconds) with acceleration and then termination of the AT within 10 seconds (Figure 3C). The epicardial catheter showed sharp potential at the successful ablation site that is suggestive of LOM muscle sleeve as an epicardial structure. There were no procedure-related complications and the AT was not inducible after ablation. Following ablation, the corrected sinus node recovery time was within normal limits. No sinus pause was observed by follow-up Holter recording. There has been no recurrence of tachycardia after more than 12 months of follow-up.
Discussion
AT originating from the epicardial LAA is rare, and its electrocardiographic characteristics are not obvious. The P wave morphology would be similar to that the AT originating from the left superior pulmonary vein and is difficult to distinguish from AT originating at the left superior pulmonary vein. The circular mapping catheter allows simultaneous mapping of multiple sites around the LAA area. Although epicardial mapping and ablation via a percutaneous approach has proven feasible and is associated with low rates of complications (5), it is not easy practically to perform epicardial mapping and ablation without 3D electroanatomical mapping system. Yamada et al. demonstrated that failed endocardial catheter ablation of AT origin may be attributed to epicardial locations and thick pectinate muscle within the LAA (3). Here, we also reported a clear epicardial arrhythmogenic focus for which multiple RF energy attempts were unable to achieve resolution from an endocardial approach. In the present case, bipolar electrogram of circular mapping catheter 5, 6 and 7, 8 which located endocardially preceded the electrograms of EPI. Initially, we attempted the endocardial RF ablation targeting at this point with the conventional ABL several times. At that time, transient AV block occurred although ongoing AT. Because of AV block without AT termination, we turned to the epicardial site. If we used the irrigated catheter and contact force monitoring system during endocardial ablation, it could have terminated the AT.
As regarding the epicardial sharp potentials, the exact abolishment site of the ATs is suggestive of LOM muscle sleeve. However, we did not confirm the LOM potentials during sinus rhythm and did not performed CS angiography to delineate the exact ablation site of LOM during procedure unfortunately. Although we performed the point by point activation mapping in the near neck of LAA under the circular mapping catheter and fluoroscopic guidance, there is still much possibility of LOM muscle sleeve as the epicardial ablation site.
In addition, there are several notable findings in the present case. The Holter recording demonstrated frequent sinus pause. This finding was similar to the tachy-bradycardia syndrome, but no evidence of sinus node dysfunction was found by electrophysiologic study after ablation of the AT. A transient AV block occurred during endocardial ablation at the neck of the LAA. A mechanism of transient AV block had not been reported previously. Lemery et al. demonstrated that approximately four sites in the LA resulted in bradycardia consistent with stimulation of ganglionated plexi. After ablation, bradycardia with ganglionated plexi stimulation was blocked in 88% of cases (6). We hypothesize several possible mechanisms for this complication: increased vagal tone due to pain or stimulation of epicardial vagal fibers or ganglionated plexi, which are densely located in the LAA area. This vagal response is not uncommon during ablation of atrial fibrillation.
In the present case, a circular mapping catheter and irrigated-tip ABL were preferred for accuracy of the epicardial LAA mapping and efficacy of energy delivery.
Although we did not performed coronary angiography to confirm the distance between the ablation site and the nearest coronary artery, close proximity of the left circumflex coronary artery branches might temper preference for irrigated RF ablation. Left phrenic nerve injury, as well as left circumflex coronary artery injury, may occur during epicardial ablation of areas near the LAA (7). Pacing at maximum output to avoid phrenic nerve stimulation should be considered prior to ablation. Although some difficulties in epicardial ablation of the LAA area occur when not using 3D electroanatomical mapping systems, a circular mapping catheter within the LAA may allow for adequate guidance for percutaneous epicardial mapping and ablation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Wagshal AB, Applebaum A, Crystal P, et al. Atrial tachycardia as the presenting sign of a left atrial appendage aneurysm. Pacing Clin Electrophysiol 2000;23:283-5. [Crossref] [PubMed]
- Yamada T, Murakami Y, Yoshida Y, et al. Electrophysiologic and electrocardiographic characteristics and radiofrequency catheter ablation of focal atrial tachycardia originating from the left atrial appendage. Heart Rhythm 2007;4:1284-91. [Crossref] [PubMed]
- Yamada T, McElderry HT, Allison JS, et al. Focal atrial tachycardia originating from the epicardial left atrial appendage. Heart Rhythm 2008;5:766-7. [Crossref] [PubMed]
- Phillips KP, Natale A, Sterba R, et al. Percutaneous pericardial instrumentation for catheter ablation of focal atrial tachycardias arising from the left atrial appendage. J Cardiovasc Electrophysiol 2008;19:430-3. [Crossref] [PubMed]
- Schweikert RA, Saliba WI, Tomassoni G, et al. Percutaneous pericardial instrumentation for endo-epicardial mapping of previously failed ablations. Circulation 2003;108:1329-35. [Crossref] [PubMed]
- Lemery R, Birnie D, Tang AS, et al. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm 2006;3:387-96. [Crossref] [PubMed]
- Sacher F, Monahan KH, Thomas SP, et al. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol 2006;47:2498-503. [Crossref] [PubMed]


