Techniques of endoscopic airway tumor treatment
Introduction
Interventional bronchoscopy mainly concerns malignant tumors (bronchogenic carcinoma in 70% of cases, metastatic malignancies in 25% of cases in our institution) but also benign tumors (5% of cases).
Lung cancer has the particularity of being tardily symptomatic, making early diagnosis a far too rare event. No screening program has yet been validated in most countries, and improvements to the early detection of metaplastic changes in selected populations are very challenging. For the same reasons, lung cancer is usually diagnosed at an advanced stage, and frequently from symptoms related to local progression of the disease, when conventional treatments offer inconstant and delayed benefits. In contrast, new bronchoscopic approaches that visualize peripheral nodules using radial-probe endobronchial ultrasonography (RP-EBUS) and electromagnetic navigation (EMN) are paving the way towards new bronchoscopic management of early-stage peripheral lung cancers.
Herein, we present an exhaustive description of the overriding role of the interventional pulmonologist in the management of pre-invasive, localized, and locally advanced lung cancer. We will detail: (I) the place of bronchoscopy in the curative treatment of benign pediculate endoluminal tumors; (II) the specific indications, results, and limitations of the bronchoscopic tools to detect and offer a curative treatment for localized neoplastic lesions, and the management of malignant central-airway obstruction; and (III) discuss the emerging endoscopic approaches used for curative treatment of peripheral lung cancer.
Curative treatment of endobronchial benign tumors
Bronchoscopic treatment of benign tumors should be restricted to strictly endoluminal polypoid tumors (<15 mm2 base), without any signs of submucosal-layer infiltration. Involvement of the airway wall must be precisely assessed by a CT-scan or ideally by more precise tools. RP-EBUS should be considered before treating such benign tumors as it can detect invasion of the cartilage layer with a sensitivity of 86% and a specificity of 100% (1). In 1995, Shah et al. reported “very good” and “good” results in 62% and 38% of patients, respectively, after bronchoscopic management of 185 benign airway tumors. The main technique used in these cases was laser resection (2). Bertoletti et al. then described the usefulness and the very good results from using cryotherapy on the implantation base of 18 strictly endoluminal and typical carcinoid tumors (3). This approach can be used for other benign tumors (4,5).
Bronchoscopic techniques for the curative management of early stage non-small-cell lung cancer (NSCLC)
Techniques for detection and local staging
Given the very poor prognosis of lung cancer, efforts have been made to improve the early detection of precancerous lesions. Indeed, 37% of severe dysplasias and 87% of in situ carcinomas evolve into invasive cancer, although less commonly in the presence of slight or moderate dysplasia (3.5%) (6). Furthermore, close surveillance is indicated for every patient that survives an initial lung cancer, as the risk of developing a second carcinoma ranges between 2% and 14% per patient per year (7,8). Different tools are available for the early detection and follow-up of these radio-occult tumors, for the screening of other endobronchial lesions before thoracic surgery, and to guide bronchoscopic curative treatments. Local staging includes evaluation of the area and thickness of the lesion.
Autofluorescence can differentiate dysplasia and cancer lesions from normal tissues based on their respective concentrations of endogenous fluorophores. The decrease in the extracellular matrix and the increased concentration of porphyrin that characterizes dysplastic tissue will thus correspond, in the autofluorescence bronchoscopy or autofluorescence imaging, to defects in fluorescence or to magenta-colored areas, respectively (9). Nevertheless, if sensitivity is good (0.9), the major pitfall of autofluorescence is its low specificity (0.56) (10); resulting in an increased number of useless biopsies (false positives). However, the results strongly depend on the predetermined definition of “positive biopsy” (i.e., 71.1% specificity for severe dysplasia and carcinoma in situ) (11).
Narrow-band imaging enables analyses of submucosal microcapillary structures, with different pathologic patterns described in dysplastic or cancerous tissues (grid dotted, tortuous, abrupt-ending blood vessels) (12,13). The advantage of this technique compared to autofluorescence imaging resides in its improved specificity (90% vs. 52%) (14).
Probe-based confocal laser endomicroscopy may further improve the diagnostic accuracy of bronchoscopy when screening pre-invasive metaplasia. The first data suggest good sensitivity (96%) and specificity (87%), which could be increased by topical instillation of exogenous fluorophores, such as acriflavine (15).
RP-EBUS can be used to evaluate the depth of invasion within the cartilaginous layer with good specificity (77%) and sensitivity (88%) (16-18). In addition, any adjacent suspected lymph nodes can be sampled by needle aspiration using linear EBUS, which is an established technique used in lung-cancer staging.
Optical coherence tomography may also help to assess parietal extension of radio-occult lesions (19).
Techniques used for the curative treatment of pre-invasive or minimally invasive lesions
Surgical resection remains the gold standard in early-stage NSCLC (20). However, bronchoscopy offers different options for the treatment of carcinoma in situ or slightly invasive endo-bronchial lesions, with good results, low morbidity, and low cost. Indeed, many patients with poor lung function or other comorbidities can then avoid surgery.
Smokers can develop multiple pre-neoplastic lesions and synchronous or metachronous squamous carcinomas within the entire upper aerodigestive tract, based on the principle of “field cancerization” (21). The main advantage of endoscopic treatment is thus the preservation of lung parenchyma and lower morbidity mortality, whereas the major risks are potential underestimation of the extension of the tumor and a lack of care regarding lymph-node metastases (22). The best candidates for bronchoscopic treatments are patients with lesions measuring <10 mm without extra-cartilaginous invasion (23). Once the histological nature and extension are assessed, different tools are available, with each having preferential indications, specific mechanisms of actions, and risks. These data are summarized in Table 1.
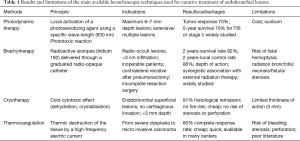
Full table
Brachytherapy
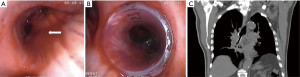
Brachytherapy is a highly localized radiation therapy that preserves healthy tissue. Radioactive isotopes (iridium-192) are delivered to the tumor through a graduated radio-opaque catheter (24). Catheters are placed through a large bronchoscope, their position controlled by fluoroscopy. The target volume is assessed based on endoscopic findings (usually helped by autofluorescence imaging) and 3D-treatment planning is then calculated from radiographic data after placement of the catheter(s). Figure 1 shows each step during treatment of endoluminal T1 squamous-cell carcinoma in a patient recused for surgery.
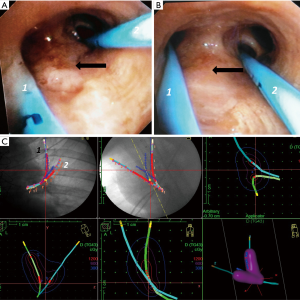
Brachytherapy should be considered as a curative treatment in radio-occult lesions, endobronchial infiltration for patients with respiratory insufficiency, controlateral recurrence after pneumonectomy, or as a complementary technique for incomplete resection surgery (25-29).
The largest cohort to date included 226 patients that had undergone brachytherapy (97% squamous cell carcinomas and had been treated with high-dose rate (HDR) brachytherapy because they had contraindications for surgery or external-beam radiation therapy. Repartition was 26% Tis, 67% T1, and 0.04% T2. A complete bronchoscopic response at 3 months was observed in 93.6% of patients. The 2- and 5-year survival rates were 57% and 29%, respectively. Fatal hemoptysis occurred in 5% of cases, bronchitis in 19.5%, with 3.5% necrosis. These complications were shown to be associated with the use of two catheters and having a distal localization (27).
A combination of external-beam radiation therapy and HDR brachytherapy has also been proposed to treat endobronchial carcinomas as an alternative to surgery, and has achieved high response and survival rates. In this study, Kawamura et al. included 16 lesions, of which 10 were treated with HDR brachytherapy (20 Gy) combined with external-beam radiation therapy (45 Gy), and 6 lesions were treated with HDR brachytherapy alone (25 Gy). The 2-year survival and local-control rates were 92.3% and 86.2%, respectively. Local recurrences were observed in only two lesions (30).
This method is expansive and remains suited for deeper lesions compared to the techniques described below. Acute side effects of this procedure include pneumothorax, bronchospasm, hemoptysis, pneumonia, and cardiac arrhythmia. Radiation bronchitis and stenosis may occur as early and delayed complications. Interventional bronchoscopy is sometimes required in cases of symptomatic stenosis (balloon dilatation, laser resection, or airway stenting) (31).
It is sometimes difficult to differentiate complications linked to tumor progression from those related to brachytherapy (32). The most serious potential side-effects from brachytherapy are fatal hemoptysis and fistula formation. Death from massive hemoptysis has been reported in up to 7% of patients (n=342), but occurred mainly within palliative settings, and this complication seems rarer in the context of minimally invasive lesions (33).
Photodynamic therapy (PDT)
The principle of bronchoscopic PDT resides in the local activation of a photosensitizing agent (most commonly a hematoporphyrin derivative) using a light source with a specific wave-length (630 nm) that induces a phototoxic reaction and cell death (24,31-33). Bronchoscopic PDT for lung cancer is a two-step procedure: photosensitization and illumination. After intravenous injection of a photosensitizer (photosensitization) and a 72-h latent period, bronchoscopic illumination is carried out. Other photosensitizers have been tried (ALA or Npe6) (34). The depth of action is 6–7 mm and the surface diffusion of light allows, in theory, treatment of slightly more extensive lesions than other techniques. Moreover, this technique can still be used when the tumor area cannot be precisely identified by narrow-band imaging or autofluorescence imaging. Depending on the number and extent of the treated lesions, PDT can be performed under local or general anesthesia, sometimes secured by rigid bronchoscopy.
Many studies have reported on the efficiency of PDT in treating central-airway obstructions (reported in the next chapter), and early stage and minimally invasive endobronchial tumors. Corti et al. reported a 5-year survival rate of 60% amongst 40 patients with CIS or minimally invasive diseases (35). Moghissi et al. collected data from PDT used for early-stage NSCLC in 13 studies (n=523 patients). Tumor response to treatment was observed in >70%. The 5-year survival amongst patients that experienced complete remission after treatment of TIS or stage-I diseases was 70%. After exclusion of deaths not related to cancer, the 5-year survival was 90% for TIS (36).
PDT is generally well tolerated. Skin photosensitivity is the most common side effect (8–28% of cases), which is usually grade I, justifying avoiding sunlight for the 6 weeks after treatment (31,36). Dyspnea may be caused by airway obstruction from necrotic debris, local airway edema, or delayed strictures, but these complications are less common in the context of early-stage disease than in a palliative situation. Fatal hemorrhage and fistulas were also very rarely reported in this indication.
Cryotherapy
Cryotherapy is the application of very low temperatures (89.5 °C) using cryogenic liquid gas (N2O, N2, CO2) to destroy tumor tissues (24,34,35,37). The mechanisms of action are associated with immediate (dehydration and cellular crystallization) (31,37-40) and delayed (apoptosis, ischemia caused by microthrombi formation) effects (31,39,41). At least three cycles of freezing and thawing are applied to the treatment area through the tip of a flexible or rigid cryoprobe. The whole surface should be treated with 5-mm spacing between each new application of the probe (31).
Deygas et al. reported 91% histological remission rate at one month in a multicenter study (n=35). Local recurrence was observed in 28% of cases within 4 years (41). Noppen et al. also reported favorable outcomes in four patients (42). Cryotherapy possesses many advantages: it is very cheap and safe (41) because the risk of airway ignition, perforation, or delayed stenosis is non-existent, and it has a good hemostatic effect (35,43). Its major pitfalls are the narrowness of the treatment area and the depth of its cytotoxic action, which is limited to 3 mm (44).
Thermocoagulation
Electrocoagulation (or electro-cautery) is a coagulation technique. A high-frequency electric current is delivered through the tumor tissue, which generates heat and destroys it (24). The probe is placed in close contact to the target tissue area and the energy setting can then be changed depending on the expected effect. A small study that included 15 <1 cm endobronchial lesions in 13 patients showed an 80% complete-response rate and no recurrences after 22 months of follow-up (45). It can be applied through flexible or rigid probes, under local or general anesthesia (46). It is inexpensive and available in all surgical wards, but some complications can occur, such as bleeding.
In conclusion, all of these methods show promising outcomes. Brachytherapy seems to be most suited to deeper lesions and PDT to extensive and/or multiples lesions, especially when the tumor area cannot be precisely identified. Nevertheless, more prospective controlled studies need to be conducted to help clinicians choose the right technique for each specific case. Associations of these different tools together with stereotactic radiotherapy or systemic treatment should also be evaluated.
Available techniques for the management of central-airway obstruction
Between 20% and 30% of lung cancers cause central-airway obstruction (CAO), resulting in dramatic alterations to quality of life and a poor prognosis. CAO can be linked to purely endoluminal tumors, extrinsic compression, or a combination of both. Multiple bronchoscopic tools are available to relieve these obstructions depending on the mechanism of the stenosis, and can be split into four main categories: (I) mechanical debulking, usually used with other techniques for intraluminal lesions; (II) thermal techniques, which have an immediate effect on severe and/or very symptomatic intraluminal stenoses; (III) thermal techniques that have a delayed effect on non-threatening intraluminal stenoses; and (IV) airway stenting for extrinsic compressions.
All these methods can be used alone or in combination (47). The precise place of each technique and the eventual superiority of one over another remains undefined, as no large clinical trials or comparative studies have been conducted. The choice of technique thus depends on the choice of the operator and the techniques available. The principles, results, and pitfalls of each technique are summarized in Table 2. Two examples of scanographic and bronchoscopic results after mechanical debulking, thermo-coagulation, and airway stenting are represented in Figures 2 and 3.
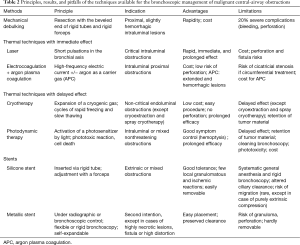
Full table
Indications
Interventional bronchoscopy is an invasive treatment and should only be used in cases of symptomatic obstruction and in the presence of a viable downstream bronchial tree and parenchyma. A CT-scan and flexible bronchoscopy are thus the two essential tools to both check the viability of the distal airways and the precise mechanism of the stenosis. Significant improvement to quality of life and symptoms can be expected for obstruction involving large airways (trachea, main bronchi, bronchus intermedius). Interventional bronchoscopy for a more distal tumor must only be proposed to control hemoptysis or draining-retentional pneumonia (48). Beyond this technical aspect, selection of the patient that will benefit most from this treatment is crucial, but is sometimes difficult. We reported some strong predictive factors for sustained efficiency in a retrospective analysis of 204 patients (49). A study is currently recruiting patients to prospectively validate these results on survival and, above all, will try to identify the predictive factors for improvement to quality of life. We hope this study will help clinicians to select the best candidates and to avoid giving an invasive treatment to others.
Modalities
Rigid bronchoscopy offers advantages in terms of airway control, the ability to easily remove large volumes of tumor, and to deploy silicone stents: this method should, in our opinion, always be favored. Treatment through a flexible bronchoscope and local anesthesia, or under slight sedation and a laryngeal mask, may be considered for small, nonthreatening, and slightly hemorrhagic tumors, when the expected time of procedure is short. When rigid bronchoscopy is chosen, different types of ventilation can be proposed: i.e., manual with a bag-valve mask, or jet ventilation (high frequency, high pressure, low volume) (48).
Techniques with an immediate effect
In cases of threatening and/or very symptomatic intra-luminal CAO, an immediate, rapid, and safe technique must be chosen. Mechanical debulking, electrocoagulation, and laser are the most widely used techniques in this context.
Mechanical debulking
Rapid debulking of intraluminal tumors can be obtained with either the beveled end of different-caliber rigid tubes or with a large clamp. However, except in cases of proximal and low-risk hemorrhage tumors, this approach usually needs to be combined with one of the thermal techniques described below, as it is associated with high rates of complications when used alone (i.e., ~20% of cases), such as pneumothorax, hemoptysis, and pneumonia (50). For example, an ideal sequence is, combined with a laser: (I) hemostasis by tumor coagulation at 30 W; (II) mechanical debulking; (III) destruction of the residual tumor tissue at 50 W; and (IV) final treatment at 20–30 W on the implantation base for a prolonged cytotoxic effect (51,52). A minimum of 15–20 supervised procedures are required to reach autonomy with a rigid bronchoscopy (53).
Thermocoagulation
This method offers rapid relief from malignant proximal intrinsic compressions. A high-frequency electric current is delivered to the endoluminal tumor obstruction through a flexible or rigid probe of varying diameter and form, such as a coagulation electrode, loop, or hot-biopsy forceps (24,31,54-56). New-generation generators can be set to different modes (55). The “soft-coagulation” mode prevents airway fire, maintaining relatively low temperatures (<200 °C). “Forced coagulation” is more risky but allows rapid debulking by tissue carbonization. Symptomatic palliation is obtained in 96% of cases and concords with functional improvement (55,56). Major complications are rare, and the particular risks of perforation and ignition are non-existent in soft-coagulation mode (55,57-59). Circumferential thermocoagulation of an airway may result in scarring stenosis (53,60,61). Thermocoagulation must therefore been considered as the first-line treatment for intra-luminal tumors as it is highly efficient, only moderately expensive, and is very safe.
Argon-plasma coagulation
The limitations of electrocoagulation include restricted access to the most apical segments and loss of efficacy when there is active bleeding. These two drawbacks are overcome by using argon as a carrier gas (non-contact method), but this technique requires an additional probe and a generator (24,31,57,62). Argon (and thus electric current) is transported to the affected vessels through the bloodstream, allowing good control of the hemorrhage (100%) (62,63), even if the origin of bleeding cannot be precisely identified (24,53,55,64,65). This makes this tool particularly suited for extensive and hemorrhagic lesions.
Laser
This method is the fastest and thus the most suitable technique for life-threatening intraluminal critical obstruction. It uses different gases (CO2, potassium titanyl phosphate, Nd:YAG, and a diode laser) to rapidly photocoagulate and destroy tumors (24,53). An immediate bronchoscopic result (92% of cases) is usually obtained, and the more proximal the tumor, the better the result (66). This re-permeabilization results in constant and significant improvement in symptoms, quality of life, arterial blood gas, and spirometric analyses (67,68). The results are globally equivalent to those reported with thermo-coagulation (55,69) but laser treatment may shorten the procedure, even though these two techniques have not been prospectively compared. The risk of airway fire only appears beyond the 0.4 FiO2 level and can thus be prevented by good communication between the anesthesiologist and pulmonologist (68,70). Hemorrhages are not rare, but can be usually efficiently controlled by cold serum, adrenaline, local instillation of terlipressin, compression with the tube, or short electrical pulses of <30 W. Severe hemoptysis only occurs in 1% of cases (52,67,70).
The major potential lethal complication, related to a high depth of action, is perforation of the tracheobronchial wall to cause a vascular fistula, gas embolism, and/or mediastinitis (70,71). This event can be avoided by respecting a tangential axis of treatment and a non-contact treatment (1 cm). Although the mortality rate is low [reported as <1% (31,68,69,71)], one pitfall of this technique is its high cost, which may be prohibitive in some centers due to the price of the generator and because of the single-use probes, with costs much higher than for electro-coagulation (54,64,69).
Cryotherapy with an immediate effect
Cryotherapy can be delivered through a flexible or rigid probe (40,72,73). This procedure is known to have delayed effects (74) and, thus, is not suitable for cases of threatening stenosis. Nevertheless, two modalities can still be considered in this context.
- Spray cryotherapy is a technique that enables low-pressure liquid nitrogen (−196 °C) to be administered, and produces immediate effects. The treatment area is extensive and the hemostatic effect is excellent. This technique, like APC, should therefore be preferentially used in cases of hemorrhagic and extensive tumors, especially when the origin of bleeding is not easily identifiable. Nevertheless, this technique still requires validation and does not appear suitable for voluminous tumors, given that this intervention has a long duration (73).
- Cryoextraction uses a large probe (2.3 mm) and is another modality used to treat obstructive stenosis without risking perforation or residual stenosis; it has a low incidence of other complications (74).
Endoscopic dilatation
Balloons placed at the center of a malignant stenosis can allow mechanical debulking and dilatation by controlled inflating (3 to 6 atm. pressure) (75-77). However, the effect is transient, and this tool should only be considered as a complement with other techniques, especially as a first step before stent placement when there is an impassable obstruction.
Microdebrider
The microdebrider is mainly used by rhino-laryngologists and involves a rotating blade and suction. This combination shortens the procedure, as tumor debris are simultaneously aspirated, and allows for rapid and efficient debulking [98% of cases (78,79)].
Techniques with a delayed effect
When there is a nonthreatening and slightly symptomatic malignant stenosis, the indications for interventional bronchoscopy must be carefully discussed. Indeed, it depends on the other therapeutic options and the probability of these conventional therapies (radiation therapy, chemotherapy) succeeding (47). Histological subtype and its supposed chemosensitivity should especially be taken into account. In this context, cryotherapy is the most attractive tool due to its low cost, good efficiency, safety, and prolonged effects. Other techniques have been reported but, in our opinion, should not be considered as first-line treatments in the context of palliative management of central-airway obstruction, like PDT or HDR brachytherapy.
Cryotherapy with a delayed effect
Except for particular cases of cryo-recanalization and spray cryotherapy (described previously), this technique is indicated in cases of non-obstructive and slightly symptomatic obstructions without acute respiratory distress, because of its delayed effects (24,55). This procedure offers good control of symptoms (especially hemoptysis), and improves quality-of-life scores, arterial blood gas, and spirometric parameters (35,37,80). A normal airway caliber can be recovered in 61–91% of cases (72,81,82), allowing for resolution of 57% and 76% of cases of total and lobar atelectasis, respectively (81). Its prolonged effect, strengthened by repetitive cryotherapy sessions, is related to an associated cytotoxic effect (74,83,84).
One major advantage of this tool is its safety. Mortality rate is low, reaching 1.2%, and is rarely directly linked to cryotherapy (80). The total complication rate is less than 10% (72,82). The risk of perforation is non-existent, as the cartilage is extremely cryo-resistant (31,72). This technique also has a low cost and a synergistic association with chemotherapy (85,86). The major caveat is its delayed effect, resulting in retention of tumoral material, which then needs bronchial aspiration performed at 1–2 days after (31,62,87).
PDT
The principle of bronchoscopic PDT resides in local activation of a photosensitizing agent (most commonly a hematoporphyrin derivative) using a light source with a specific wave-length (630 nm), which induces a phototoxic reaction and cell death (24,31,38,43,88). Like cryotherapy, PDT achieves good but delayed improvement, and is therefore contraindicated in cases of critical obstruction. This technique is particularly effective in controlling hemoptysis (99%) and dyspnea, and offers good bronchoscopic results and significant functional improvement (89-91). A synergistic effect with radiation therapy is probable (92).
However, this technique has major limitations. The drug is eliminated from most tissues within 72 hours, yet remains preferentially stored in the skin, liver, spleen (and in malignant cells), explaining the phototoxic reactions observed in 5–28% of patients. Avoidance to sunlight is recommended during the 6 weeks after treatment (52,91); however, this preventive measure appears to be overly restrictive in a palliative context. In addition, a second bronchoscopy must be performed at 1–2 days later to remove necrotic tumor tissue and again at 5–7 days after to expose residual tissue to a second illumination (83,88,89,93,94). Furthermore, hemoptysis is a relatively frequent complication, reported in 18% of cases, of which 2.2% are fatal (89); the mortality rate is not negligible, reaching 9% during the first month (91).
Brachytherapy
Although brachytherapy, as described previously, still constitutes an interesting option for early-stage lung cancer, in our opinion, it should no longer be used for the palliative treatment of malignant obstructions. Symptomatic and functional improvements have been reported in older studies, with delayed efficiency, but the risk of severe complications has been major, reported at between 13 and 20% (33,95). Up to 7% of patients died after massive hemoptysis (33). Radiation bronchitis, which frequently exhibits a fatal evolution due to bronchial necrosis followed by abscess formation, affected 14–35% of patients (96,97). Nevertheless, experimental groups have reported good results and slightly better tolerance to this treatment. Interestingly, in a very large cohort (n=648) of patients divided into two groups that received a single fraction of 10 or 22.5 Gy in three fractions once a week, the clinical improvement was globally equivalent (98).
Local instillation of chemotherapy and gene therapy
Local intra-tumoral injection of chemotherapy has been attempted in the context of malignant central-airway obstruction. Celikoğlu et al. reported re-permeabilization in more than 80% of patients using different cytotoxic agents (5FU, mitomycin, methotrexate, bleomycin, mitoxantrone, cisplatin) (99-101). More recently, Mehta et al. also described good bronchoscopic results (defined as >50% reduction in airway stenosis from baseline) after intratumoral injection of cisplatin in 15/21 patients (102). Khan et al. obtained complete PET/CT remission of locally recurrent lung cancer after an EBUS-guided transbronchial needle-injection of cisplatin in a hilar lymph node (103).
Another promising approach is the bronchoscopic delivery of a recombinant adenovirus that carries wild-type p53 into patients with NSCLC and that harbor the p53 mutation. After monthly injections, 50% (6/12) of patients had airway obstruction improved by >25%, and 25% of patients showed a partial response (104). Combined with external radiation therapy (60 Gy), this strategy enabled 63% of cases to obtain local and complete-response rates (105).
Stents
Airway stenting is the only procedure available for the relief of extrinsic compression or trachea-esophageal fistulas (75,106,107). It has been widely used since Dumon designed a silicone stent, derived from Montgomery’s T-shaped tracheal stent (which required a tracheotomy) (83,108). Self-expandable metallic stents dedicated to airways were concomitantly developed, after the transient use of endovascular stents (Gianturco), which were associated with a high risk of ischemic mucosal necrosis and thus of perforation (109). The ideal prosthesis should (I) be cost-effective; (II) be easy to place and remove; (III) not migrate; (IV) be rigid enough to resist airway compression, yet still flexible enough to mimic airway physiology; (V) not impair mucociliary clearance, and (VI) not induce granulomatous reactions (38,106,110). The most widely used stents are represented in Figure 4.
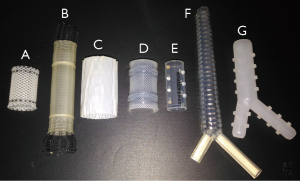
Experts suggest that a minimum of 5–10 procedures per year after 10–20 supervised procedures are required to maintain competence of airway stenting (24,55).
Silicone stents
In the context of malignant central-airway obstruction, we recommend silicone stents as the first-line treatment (109,111). Indeed, they are easy to place and remove, they are well tolerated, have a marked vault effect, and cause few granulomatous reactions (24,106,108). The Dumon (Tracheobronxane®, Novatech, La Ciotat, France) is the most widely used stent (108). Alternatives are the Polyflex® stent (silicone expandable stent) (Boston Scientific, Natick, MA, USA), the Hood stent® (Hood Laboratories, Pembroke, MA, USA), and the Noppen stent (Reynders Medical Supply, Lennik, Belgium).
The Dynamic Freitag® (Rüsch, Kernen, Germany) stent has a flexible posterior wall that mimics the physiological behavior caused during coughing. This stent should theoretically be associated with a lower risk of granuloma because of the more homogeneous distribution of pressures (112,113). Silicone stents can be straight or bifurcated for stenoses that involve the carina or primary right carina (Oki stent) (106,114). The diameter and size are chosen based on scanographic and per-operative bronchoscopic data, which are then adjusted by cutting (37,75,108,115-117).
Symptoms and quality of life are immediately improved in the vast majority of cases (52,75). Symptomatic granuloma is rare (1%, n=306) (10,118). Migration risk mainly concerns short and purely extrinsic stenoses (24,38,118,119) and immediate subglottic stenoses are relatively rare in cases of obstructions of malignant origin [2–6% (119) of cases vs. 18% with benign stenoses (120)]. The overall complication rate has been reported as 9.4% (52).
The few limitations of silicone stents compared to self-expandable metallic stents are caused by a narrower internal diameter due to wall thickness, which results in altered mucociliary clearance (38,106): i.e., obstruction by secretions (14% of patients) (64,111), favored by bacterial colonization (121), which can be avoided by nebulizations and good hydration (24).
Self-expandable metallic and hybrid stents
Self-expandable metallic stents (Ultraflex®, Boston Scientific; Alveolus®, Charlotte, NC, USA, Aerstent® Leufen, Germany) can be placed under video-bronchoscopic or radioscopic guidance (122). Their advantages include a lower risk of migration, better preserved mucociliary clearance, and larger internal diameter (106). They also offer rapid and good control of symptoms, and improved quality-of-life scores and spirometric parameters (85,107,110). However, even if these stents can be placed under flexible bronchoscopy, we do not recommend this approach (except in cases of intubated patients with no access to rigid bronchoscopy), as the control of obstructive or hemorrhagic complications is more rapid under rigid bronchoscopy. Self-expandable coated stents are particularly suitable for trachea esophageal fistulas, for tight and highly distorted stenoses, and for highly necrotic stenoses, as they avoid the need to bypass the stenosis with a rigid tube (which increases the risk of perforation) (107,109,118,123).
Major limitations include the risk of granulomatous reactions at the extremities (118); epithelialization with incorporation into the mucosa, thus rendering the stent difficult to remove after 3–6 weeks (116); a weaker vault effect (111); and an increased risk of perforation (109). The rate of significant complications is often high for these stents, reaching 16% in the first month and 13% afterwards (124). Early complications include pneumothorax, pneumonia, and migration (118). Hemoptysis and infections are seen in 10% of patients (125). At later stages, symptomatic granuloma formation is observed in 15% (118) to 27% of cases (126).
Drug-eluting stents
These may be an interesting way to prevent granuloma and malignant-tissue formation. This approach has mostly been evaluated in gastrointestinal endoscopy, with anticancer or antiproliferative (mTOR inhibitors) agents (127). A biodegradable cisplatin-eluting stent has been designed, dedicated to the central airways, but has not yet been tested in humans (128). The main pitfall of such a prosthesis may be an enhanced risk of a fistula caused by its antiproliferative action.
Bronchoscopic management of endobronchial metastases
Endobronchial metastasis of other solid tumors is relatively rare; it usually occurs tardily after the initial diagnosis (median 56 months) (129). A classification has been proposed to described their mechanisms: (I) type I is a directly endoluminal metastasis; (II) type II corresponds to bronchial invasion by a parenchymal lesion; (III) type III is the result of bronchial invasion by a mediastinal or hilar lymph node; and (IV) type IV are peripheral lesions extending along the proximal bronchus (129). Most frequently involved malignancies are breast, colon, and renal-cell carcinomas (130).
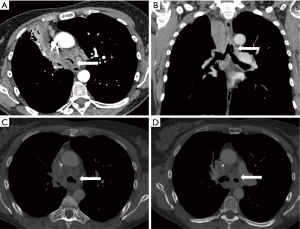
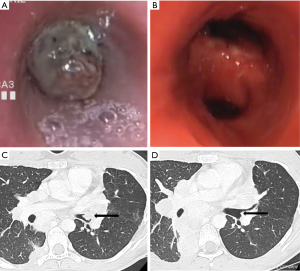
In a cohort of 24 patients undergoing bronchoscopic management of endobronchial metastasis from colorectal cancer, dramatic symptomatic and functional improvement was observed and median survival after the procedure was 14 months (131). Dalar et al. reported results from the bronchoscopic management of 20 procedures in nine patients suffering from endoluminal metastases of renal-cell carcinoma. The procedure was efficient in most cases with median survival after the intervention of 8.7 months. The operator facing this histology has to be particularly prepared to face hemoptysis (60% of hemorrhagic procedures in this cohort) (12/20) (132). Bronchoscopic treatment of airway metastases from a melanoma is also usually efficient and does not show any technical particularities. Median survival after the procedure is 6 months (n=18) (133). Figure 5 represents the results after bronchoscopic management of a type-I airway metastasis.
Emerging bronchoscopic techniques for the management of peripheral lung cancer
Although surgery remains the gold-standard treatment for early-stage lung cancer, alternative options have been developed to treat the most vulnerable and inoperable patients. Stereotactic body-radiation therapy has been evaluated the most and, thus, is the non-surgical treatment of choice, followed by percutaneous thermoablation. New bronchoscopic techniques that access distal nodules, mainly RP-EBUS, are paving the way towards new options to treat early-stage peripheral cancer (134).
Trans-bronchial CT-guided brachytherapy has been described in a few cases of peripheral lung cancer. Barium was injected through a catheter, placed under bronchoscopic control, to check its position with a CT-scan. Lateral and frontal X-rays allowed brachytherapy to be planned and iridium-92 was delivered using the HDR after-loading system. One of the two patients experienced a 75% decrease in tumor burden after a single dose of 15 Gy whereas the other patient did not respond after three fractions given at weekly intervals (total 24 Gy) (135). HDR brachytherapy (three fractions of 5 Gy) was also delivered to a patient through a catheter that was placed using both EMN and RP-EBUS: this resulted in a durable partial response and a complete histological response in RP-EBUS-guided biopsy specimens at 12 months (136).
Because radiofrequency ablation has a high risk for pneumothorax, bronchoscopy-guided radiofrequency ablation has been reported as a safe alternative for selected patients. Amongst 23 peripheral lung cancers in 20 patients, 11 tumors showed significantly reduced tumor size and eight cases showed stability, resulting in a disease-control rate of 82.6% and a 5-year overall survival of 61.5%. Given these interesting results, we can anticipate a role for EMN and/or RP-EBUS to guide and thus improve the precision of this new strategy.
Other techniques should be evaluated for the treatment of peripheral early lung cancers, such as cryotherapy or PDT, which would be delivered through small cryoprobes/laser fibers after confirmation of the tumor location by EMN of RP-EBUS.
Finally, bronchoscopy has been evaluated as a tool for the placement of fiducial markers to guide real-time tumor-tracking radiation therapy for peripheral lung nodules (137). This option could also be improved by EMN of RP-EBUS.
In conclusion, the interventional pulmonologist holds an increasingly important place within each step of managing lung cancer. The early detection of pre-invasive endo-bronchial lesions is a current challenge, justifying the recent development of several tools with increased diagnostic accuracy and of mini-invasive techniques that have curative treatments.
In more advanced stages of lung cancer, interventional bronchoscopy offers many different possibilities to relieve central-airway obstruction when it is associated with a poor prognosis, and it greatly increases quality of life. The choice of technique ultimately depends on both the mechanism and the respiratory repercussions of the stenosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kurimoto N, Murayama M, Yoshioka S, et al. Assessment of usefulness of endobronchial ultrasonography in determination of depth of tracheobronchial tumor invasion. Chest 1999;115:1500-6. [Crossref] [PubMed]
- Shah H, Garbe L, Nussbaum E, et al. Benign tumors of the tracheobronchial tree. Endoscopic characteristics and role of laser resection. Chest 1995;107:1744-51. [Crossref] [PubMed]
- Bertoletti L, Elleuch R, Kaczmarek D, et al. Bronchoscopic cryotherapy treatment of isolated endoluminal typical carcinoid tumor. Chest 2006;130:1405-11. [Crossref] [PubMed]
- Rabeau A, Mazieres J, Hermant C, et al. Bronchoscopic Multimodal Management of Tracheal Neurofibroma. J Bronchology Interv Pulmonol 2016;23:340-2. [Crossref] [PubMed]
- Guibert N, Mazieres J, Didier A, et al. Tracheal Glomangioleiomyoma Treated by Multimodal Interventional Bronchoscopy. Ann Thorac Surg 2016;101:1591-4. [Crossref] [PubMed]
- Bota S, Auliac JB, Paris C, et al. Follow-up of bronchial precancerous lesions and carcinoma in situ using fluorescence endoscopy. Am J Respir Crit Care Med 2001;164:1688-93. [Crossref] [PubMed]
- Cortese DA, Pairolero PC, Bergstralh EJ, et al. Roentgenographically occult lung cancer. A ten-year experience. J Thorac Cardiovasc Surg 1983;86:373-80. [PubMed]
- Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst 1998;90:1335-45. [Crossref] [PubMed]
- Zellweger M, Grosjean P, Goujon D, et al. In vivo autofluorescence spectroscopy of human bronchial tissue to optimize the detection and imaging of early cancers. J Biomed Opt 2001;6:41-51. [Crossref] [PubMed]
- Chen W, Gao X, Tian Q, et al. A comparison of autofluorescence bronchoscopy and white light bronchoscopy in detection of lung cancer and preneoplastic lesions: a meta-analysis. Lung Cancer 2011;73:183-8. [Crossref] [PubMed]
- Ueno K, Kusunoki Y, Imamura F, et al. Clinical experience with autofluorescence imaging system in patients with lung cancers and precancerous lesions. Respiration 2007;74:304-8. [Crossref] [PubMed]
- Shibuya K, Hoshino H, Chiyo M, et al. Subepithelial vascular patterns in bronchial dysplasias using a high magnification bronchovideoscope. Thorax 2002;57:902-7. [Crossref] [PubMed]
- Zaric B, Perin B, Stojsic V, et al. Relation between vascular patterns visualized by Narrow Band Imaging (NBI) videobronchoscopy and histological type of lung cancer. Med Oncol 2013;30:374. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Anantham D, et al. Narrow-band imaging bronchoscopy increases the specificity of bronchoscopic early lung cancer detection. J Thorac Oncol 2009;4:1060-5. [Crossref] [PubMed]
- Fuchs FS, Zirlik S, Hildner K, et al. Confocal laser endomicroscopy for diagnosing lung cancer in vivo. Eur Respir J 2013;41:1401-8. [Crossref] [PubMed]
- Takahashi H, Handa M, Oyaizu A, et al. Ultrasonographical approach for the diagnosis on the depth of invasion in early bronchogenic squamous cell carcinoma. Kyobu Geka 2001;54:907-12. [PubMed]
- Takahashi H, Sagawa M, Sato M, et al. A prospective evaluation of transbronchial ultrasonography for assessment of depth of invasion in early bronchogenic squamous cell carcinoma. Lung Cancer 2003;42:43-9. [Crossref] [PubMed]
- Miyazu Y, Miyazawa T, Kurimoto N, et al. Endobronchial ultrasonography in the assessment of centrally located early-stage lung cancer before photodynamic therapy. Am J Respir Crit Care Med 2002;165:832-7. [Crossref] [PubMed]
- Michel RG, Kinasewitz GT, Fung KM, et al. Optical coherence tomography as an adjunct to flexible bronchoscopy in the diagnosis of lung cancer: a pilot study. Chest 2010;138:984-8. [Crossref] [PubMed]
- Kennedy TC, McWilliams A, Edell E, et al. Bronchial intraepithelial neoplasia/early central airways lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:221S-233S.
- Mohan M, Jagannathan N. Oral field cancerization: an update on current concepts. Oncol Rev 2014;8:244. [Crossref] [PubMed]
- Nagamoto N, Saito Y, Ohta S, et al. Relationship of lymph node metastasis to primary tumor size and microscopic appearance of roentgenographically occult lung cancer. Am J Surg Pathol 1989;13:1009-13. [Crossref] [PubMed]
- Fujimura S, Sakurada A, Sagawa M, et al. A therapeutic approach to roentgenographically occult squamous cell carcinoma of the lung. Cancer 2000;89:2445-8. [Crossref] [PubMed]
- Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J 2002;19:356-73. [PubMed]
- Hennequin C, Bleichner O, Trédaniel J, et al. Long-term results of endobronchial brachytherapy: A curative treatment? Int J Radiat Oncol Biol Phys 2007;67:425-30. [Crossref] [PubMed]
- Pérol M, Caliandro R, Pommier P, et al. Curative irradiation of limited endobronchial carcinomas with high-dose rate brachytherapy. Results of a pilot study. Chest 1997;111:1417-23. [Crossref] [PubMed]
- Aumont-le Guilcher M, Prevost B, Sunyach MP, et al. High-dose-rate brachytherapy for non-small-cell lung carcinoma: a retrospective study of 226 patients. Int J Radiat Oncol Biol Phys 2011;79:1112-6. [Crossref] [PubMed]
- Vonk-Noordegraaf A, Postmus PE, Sutedja TG. Bronchoscopic treatment of patients with intraluminal microinvasive radiographically occult lung cancer not eligible for surgical resection: a follow-up study. Lung Cancer 2003;39:49-53. [Crossref] [PubMed]
- Skowronek J. Brachytherapy in the treatment of lung cancer - a valuable solution. J Contemp Brachytherapy 2015;7:297-311. [Crossref] [PubMed]
- Kawamura H, Ebara T, Katoh H, et al. Long-term results of curative intraluminal high dose rate brachytherapy for endobronchial carcinoma. Radiat Oncol 2012;7:112. [Crossref] [PubMed]
- Vergnon JM, Huber RM, Moghissi K. Place of cryotherapy, brachytherapy and photodynamic therapy in therapeutic bronchoscopy of lung cancers. Eur Respir J 2006;28:200-18. [Crossref] [PubMed]
- Hennequin C, Tredaniel J, Chevret S, et al. Predictive factors for late toxicity after endobronchial brachytherapy: a multivariate analysis. Int J Radiat Oncol Biol Phys 1998;42:21-7. [Crossref] [PubMed]
- Speiser BL, Spratling L. Remote afterloading brachytherapy for the local control of endobronchial carcinoma. Int J Radiat Oncol Biol Phys 1993;25:579-87. [Crossref] [PubMed]
- Usuda J, Ichinose S, Ishizumi T, et al. Outcome of photodynamic therapy using NPe6 for bronchogenic carcinomas in central airways >1.0 cm in diameter. Clin Cancer Res 2010;16:2198-204. [Crossref] [PubMed]
- Corti L, Toniolo L, Boso C, et al. Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med 2007;39:394-402. [Crossref] [PubMed]
- Moghissi K, Dixon K. Is bronchoscopic photodynamic therapy a therapeutic option in lung cancer? Eur Respir J 2003;22:535-41. [Crossref] [PubMed]
- Dasgupta A, Dolmatch BL, Abi-Saleh WJ, et al. Self-expandable metallic airway stent insertion employing flexible bronchoscopy: preliminary results. Chest 1998;114:106-9. [Crossref] [PubMed]
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. [Crossref] [PubMed]
- Asimakopoulos G, Beeson J, Evans J, et al. Cryosurgery for malignant endobronchial tumors: analysis of outcome. Chest 2005;127:2007-14. [Crossref] [PubMed]
- Vergnon JM, Schmitt T, Alamartine E, et al. Initial combined cryotherapy and irradiation for unresectable non-small cell lung cancer. Preliminary results. Chest 1992;102:1436-40. [Crossref] [PubMed]
- Deygas N, Froudarakis M, Ozenne G, et al. Cryotherapy in early superficial bronchogenic carcinoma. Chest 2001;120:26-31. [Crossref] [PubMed]
- Noppen M, Meysman M, Van Herreweghe R, et al. Bronchoscopic cryotherapy: preliminary experience. Acta Clin Belg 2001;56:73-7. [Crossref] [PubMed]
- McCaughan JS, Hawley PC, Brown DG, et al. Effect of light dose on the photodynamic destruction of endobronchial tumors. Ann Thorac Surg 1992;54:705-11. [Crossref] [PubMed]
- Homasson JP, Thiery JP, Angebault M, et al. The operation and efficacy of cryosurgical, nitrous oxide-driven cryoprobe. I. Cryoprobe physical characteristics: their effects on cell cryodestruction. Cryobiology 1994;31:290-304. [Crossref] [PubMed]
- van Boxem TJ, Venmans BJ, Schramel FM, et al. Radiographically occult lung cancer treated with fibreoptic bronchoscopic electrocautery: a pilot study of a simple and inexpensive technique. Eur Respir J 1998;11:169-72. [Crossref] [PubMed]
- Daniels JMA, Sutedja TG. Detection and minimally invasive treatment of early squamous lung cancer. Ther Adv Med Oncol 2013;5:235-48. [Crossref] [PubMed]
- Guibert N, Mazieres J, Marquette CH, et al. Integration of interventional bronchoscopy in the management of lung cancer. Eur Respir Rev 2015;24:378-91. [Crossref] [PubMed]
- Biro P, Layer M, Becker HD, et al. Influence of airway-occluding instruments on airway pressure during jet ventilation for rigid bronchoscopy. Br J Anaesth 2000;85:462-5. [Crossref] [PubMed]
- Guibert N, Mazieres J, Lepage B, et al. Prognostic factors associated with interventional bronchoscopy in lung cancer. Ann Thorac Surg 2014;97:253-9. [Crossref] [PubMed]
- Mathisen DJ, Grillo HC. Endoscopic relief of malignant airway obstruction. Ann Thorac Surg 1989;48:469-73; discussion 473-5. [Crossref] [PubMed]
- Macha HN, Becker KO, Kemmer HP. Pattern of failure and survival in endobronchial laser resection. A matched pair study. Chest 1994;105:1668-72. [Crossref] [PubMed]
- Cavaliere S, Venuta F, Foccoli P, et al. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest 1996;110:1536-42. [Crossref] [PubMed]
- Ernst A, Silvestri GA, Johnstone D, et al. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest 2003;123:1693-717. [Crossref] [PubMed]
- van Boxem TJ, Westerga J, Venmans BJ, et al. Tissue effects of bronchoscopic electrocautery: bronchoscopic appearance and histologic changes of bronchial wall after electrocautery. Chest 2000;117:887-91. [Crossref] [PubMed]
- Tremblay A, Marquette CH. Endobronchial electrocautery and argon plasma coagulation: a practical approach. Can Respir J 2004;11:305-10. [Crossref] [PubMed]
- Petrou M, Kaplan D, Goldstraw P. Bronchoscopic diathermy resection and stent insertion: a cost effective treatment for tracheobronchial obstruction. Thorax 1993;48:1156-9. [Crossref] [PubMed]
- Hollingsworth HM. Wheezing and stridor. Clin Chest Med 1987;8:231-40. [PubMed]
- Horinouchi H, Miyazawa T, Takada K, et al. Safety Study of Endobronchial Electrosurgery for Tracheobronchial Lesions: Multicenter Prospective Study. J Bronchol 2008;15:228-32. [Crossref]
- Hooper RG, Jackson FN. Endobronchial electrocautery. Chest 1988;94:595-8. [Crossref] [PubMed]
- Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest 2000;118:516-21. [Crossref] [PubMed]
- Verkindre C, Brichet A, Maurage CA, et al. Morphological changes induced by extensive endobronchial electrocautery. Eur Respir J 1999;14:796-9. [Crossref] [PubMed]
- Okada S, Yamauchi H, Ishimori S, et al. Endoscopic surgery with a flexible bronchoscope and argon plasma coagulation for tracheobronchial tumors. J Thorac Cardiovasc Surg 2001;121:180-2. [Crossref] [PubMed]
- Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7. [Crossref] [PubMed]
- Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J 2006;27:1258-71. [Crossref] [PubMed]
- Nihei K, Ishikura S, Kawashima M, et al. Short-course palliative radiotherapy for airway stenosis in non-small cell lung cancer. Int J Clin Oncol 2002;7:284-8. [PubMed]
- Cavaliere S, Foccoli P, Farina PL. Nd:YAG laser bronchoscopy. A five-year experience with 1,396 applications in 1,000 patients. Chest 1988;94:15-21. [Crossref] [PubMed]
- Venuta F, Rendina EA, De Giacomo T, et al. Nd:YAG laser resection of lung cancer invading the airway as a bridge to surgery and palliative treatment. Ann Thorac Surg 2002;74:995-8. [Crossref] [PubMed]
- Kvale PA, Eichenhorn MS, Radke JR, et al. YAG laser photoresection of lesions obstructing the central airways. Chest 1985;87:283-8. [Crossref] [PubMed]
- Boxem Tv, Muller M, Venmans B, et al. Nd-YAG laser vs bronchoscopic electrocautery for palliation of symptomatic airway obstruction: a cost-effectiveness study. Chest 1999;116:1108-12. [Crossref] [PubMed]
- Dumon JF, Shapshay S, Bourcereau J, et al. Principles for safety in application of neodymium-YAG laser in bronchology. Chest 1984;86:163-8. [Crossref] [PubMed]
- Brutinel WM, Cortese DA, McDougall JC, et al. A two-year experience with the neodymium-YAG laser in endobronchial obstruction. Chest 1987;91:159-65. [Crossref] [PubMed]
- Maiwand MO, Asimakopoulos G. Cryosurgery for lung cancer: clinical results and technical aspects. Technol Cancer Res Treat 2004;3:143-50. [Crossref] [PubMed]
- Finley DJ, Dycoco J, Sarkar S, et al. Airway spray cryotherapy: initial outcomes from a multiinstitutional registry. Ann Thorac Surg 2012;94:199-203; discussion 203-4. [Crossref] [PubMed]
- Hetzel M, Hetzel J, Schumann C, et al. Cryorecanalization: a new approach for the immediate management of acute airway obstruction. J Thorac Cardiovasc Surg 2004;127:1427-31. [Crossref] [PubMed]
- Bolliger CT, Probst R, Tschopp K, et al. Silicone stents in the management of inoperable tracheobronchial stenoses. Indications and limitations. Chest 1993;104:1653-9. [Crossref] [PubMed]
- Hautmann H, Gamarra F, Pfeifer KJ, et al. Fiberoptic bronchoscopic balloon dilatation in malignant tracheobronchial disease: indications and results. Chest 2001;120:43-9. [Crossref] [PubMed]
- Shitrit D, Kuchuk M, Zismanov V, et al. Bronchoscopic balloon dilatation of tracheobronchial stenosis: long-term follow-up. Eur J Cardiothorac Surg 2010;38:198-202. [Crossref] [PubMed]
- Casal RF, Iribarren J, Eapen G, et al. Safety and effectiveness of microdebrider bronchoscopy for the management of central airway obstruction. Respirology 2013;18:1011-5. [Crossref] [PubMed]
- Lunn W, Garland R, Ashiku S, et al. Microdebrider bronchoscopy: a new tool for the interventional bronchoscopist. Ann Thorac Surg 2005;80:1485-8. [Crossref] [PubMed]
- Maiwand MO. Cryotherapy for advanced carcinoma of the trachea and bronchi. Br Med J Clin Res Ed 1986;293:181-2. [Crossref] [PubMed]
- Marasso A, Gallo E, Massaglia GM, et al. Cryosurgery in bronchoscopic treatment of tracheobronchial stenosis. Indications, limits, personal experience. Chest 1993;103:472-4. [Crossref] [PubMed]
- Maiwand MO. The role of cryosurgery in palliation of tracheo-bronchial carcinoma. Eur J Cardiothorac Surg 1999;15:764-8. [Crossref] [PubMed]
- Montgomery WW. T-tube tracheal stent. Arch Otolaryngol 1965;82:320-1. [Crossref] [PubMed]
- Homasson JP, Renault P, Angebault M, et al. Bronchoscopic cryotherapy for airway strictures caused by tumors. Chest 1986;90:159-64. [Crossref] [PubMed]
- Razi SS, Lebovics RS, Schwartz G, et al. Timely airway stenting improves survival in patients with malignant central airway obstruction. Ann Thorac Surg 2010;90:1088-93. [Crossref] [PubMed]
- Homasson JP, Pecking A, Roden S, et al. Tumor fixation of bleomycin labeled with 57 cobalt before and after cryotherapy of bronchial carcinoma. Cryobiology 1992;29:543-8. [Crossref] [PubMed]
- Hauck RW, Lembeck RM, Emslander HP, et al. Implantation of Accuflex and Strecker stents in malignant bronchial stenoses by flexible bronchoscopy. Chest 1997;112:134-44. [Crossref] [PubMed]
- Moghissi K, Dixon K, Thorpe JA, et al. Photodynamic therapy (PDT) in early central lung cancer: a treatment option for patients ineligible for surgical resection. Thorax 2007;62:391-5. [Crossref] [PubMed]
- Moghissi K, Dixon K, Stringer M, et al. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: experience of 100 cases. Eur J Cardiothorac Surg 1999;15:1-6. [Crossref] [PubMed]
- Vincent RG, Dougherty TJ, Rao U, et al. Photoradiation therapy in advanced carcinoma of the trachea and bronchus. Chest 1984;85:29-33. [Crossref] [PubMed]
- Minnich DJ, Bryant AS, Dooley A, et al. Photodynamic laser therapy for lesions in the airway. Ann Thorac Surg 2010;89:1744-8; discussion 1748-9.
- Lam S, Kostashuk EC, Coy EP, et al. A randomized comparative study of the safety and efficacy of photodynamic therapy using Photofrin II combined with palliative radiotherapy versus palliative radiotherapy alone in patients with inoperable obstructive non-small cell bronchogenic carcinoma. Photochem Photobiol 1987;46:893-7. [Crossref] [PubMed]
- Maziak DE, Markman BR, MacKay JA, et al. Photodynamic therapy in nonsmall cell lung cancer: a systematic review. Ann Thorac Surg 2004;77:1484-91. [Crossref] [PubMed]
- Diaz-Jiménez JP, Martínez-Ballarín JE, Llunell A, et al. Efficacy and safety of photodynamic therapy versus Nd-YAG laser resection in NSCLC with airway obstruction. Eur Respir J 1999;14:800-5. [Crossref] [PubMed]
- Mehta M, Shahabi S, Jarjour N, et al. Effect of endobronchial radiation therapy on malignant bronchial obstruction. Chest 1990;97:662-5. [Crossref] [PubMed]
- Chella A, Ambrogi MC, Ribechini A, et al. Combined Nd-YAG laser/HDR brachytherapy versus Nd-YAG laser only in malignant central airway involvement: a prospective randomized study. Lung Cancer 2000;27:169-75. [Crossref] [PubMed]
- Trédaniel J, Hennequin C, Zalcman G, et al. Prolonged survival after high-dose rate endobronchial radiation for malignant airway obstruction. Chest 1994;105:767-72. [Crossref] [PubMed]
- Skowronek J, Kubaszewska M, Kanikowski M, et al. HDR endobronchial brachytherapy (HDRBT) in the management of advanced lung cancer--comparison of two different dose schedules. Radiother Oncol 2009;93:436-40. [Crossref] [PubMed]
- Celikoğlu SI, Karayel T, Demirci S, et al. Direct injection of anti-cancer drugs into endobronchial tumours for palliation of major airway obstruction. Postgrad Med J 1997;73:159-62. [Crossref] [PubMed]
- Celikoğlu F, Celikoğlu SI. Intratumoural chemotherapy with 5-fluorouracil for palliation of bronchial cancer in patients with severe airway obstruction. J Pharm Pharmacol 2003;55:1441-8. [Crossref] [PubMed]
- Celikoglu F, Celikoglu SI, York AM, et al. Intratumoral administration of cisplatin through a bronchoscope followed by irradiation for treatment of inoperable non-small cell obstructive lung cancer. Lung Cancer 2006;51:225-36. [Crossref] [PubMed]
- Mehta HJ, Begnaud A, Penley AM, et al. Restoration of Patency to Central Airways Occluded by Malignant Endobronchial Tumors Using Intratumoral Injection of Cisplatin. Ann Am Thorac Soc 2015;12:1345-50. [Crossref] [PubMed]
- Khan F, Anker CJ, Garrison G, et al. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent non-small cell lung cancer. Ann Am Thorac Soc 2015;12:101-4. [Crossref] [PubMed]
- Weill D, Mack M, Roth J, et al. Adenoviral-mediated p53 gene transfer to non-small cell lung cancer through endobronchial injection. Chest 2000;118:966-70. [Crossref] [PubMed]
- Swisher SG, Roth JA, Komaki R, et al. Induction of p53-regulated genes and tumor regression in lung cancer patients after intratumoral delivery of adenoviral p53 (INGN 201) and radiation therapy. Clin Cancer Res 2003;9:93-101. [PubMed]
- Chin CS, Litle V, Yun J, et al. Airway stents. Ann Thorac Surg 2008;85:S792-6. [Crossref] [PubMed]
- Miyazawa T, Yamakido M, Ikeda S, et al. Implantation of ultraflex nitinol stents in malignant tracheobronchial stenoses. Chest 2000;118:959-65. [Crossref] [PubMed]
- Dumon JF. A dedicated tracheobronchial stent. Chest 1990;97:328-32. [Crossref] [PubMed]
- Puma F, Farabi R, Urbani M, et al. Long-term safety and tolerance of silicone and self-expandable airway stents: an experimental study. Ann Thorac Surg 2000;69:1030-4. [Crossref] [PubMed]
- Mroz RM, Kordecki K, Kozlowski MD, et al. Severe respiratory distress caused by central airway obstruction treated with self-expandable metallic stents. J Physiol Pharmacol 2008;59 Suppl 6:491-7. [PubMed]
- Saji H, Furukawa K, Tsutsui H, et al. Outcomes of airway stenting for advanced lung cancer with central airway obstruction. Interact Cardiovasc Thorac Surg 2010;11:425-8. [Crossref] [PubMed]
- Freitag L, Eicker R, Linz B, et al. Theoretical and experimental basis for the development of a dynamic airway stent. Eur Respir J 1994;7:2038-45. [PubMed]
- Freitag L, Tekolf E, Stamatis G, et al. Clinical evaluation of a new bifurcated dynamic airway stent: a 5-year experience with 135 patients. Thorac Cardiovasc Surg 1997;45:6-12. [Crossref] [PubMed]
- Oki M, Saka H. New dedicated bifurcated silicone stent placement for stenosis around the primary right carina. Chest 2013;144:450-5. [Crossref] [PubMed]
- Lee KS, Boiselle PM. Update on multidetector computed tomography imaging of the airways. J Thorac Imaging 2010;25:112-24. [Crossref] [PubMed]
- Boiselle PM, Ernst A. Recent advances in central airway imaging. Chest 2002;121:1651-60. [Crossref] [PubMed]
- Oki M, Saka H. Thin bronchoscope for evaluating stenotic airways during stenting procedures. Respiration 2011;82:509-14. [Crossref] [PubMed]
- Husain SA, Finch D, Ahmed M, et al. Long-term follow-up of ultraflex metallic stents in benign and malignant central airway obstruction. Ann Thorac Surg 2007;83:1251-6. [Crossref] [PubMed]
- Neyman K, Sundset A, Espinoza A, et al. Survival and complications after interventional bronchoscopy in malignant central airway obstruction: a single-center experience. J Bronchology Interv Pulmonol 2011;18:233-8. [Crossref] [PubMed]
- Martinez-Ballarin JI, Diaz-Jimenez JP, Castro MJ, et al. Silicone stents in the management of benign tracheobronchial stenoses. Tolerance and early results in 63 patients. Chest 1996;109:626-9. [Crossref] [PubMed]
- Noppen M, Piérard D, Meysman M, et al. Bacterial colonization of central airways after stenting. Am J Respir Crit Care Med 1999;160:672-7. [Crossref] [PubMed]
- Du Rand IA, Barber PV, Goldring J, et al. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax 2011;66 Suppl 3:iii1-21. [PubMed]
- Hürtgen M, Herber SC. Treatment of malignant tracheoesophageal fistula. Thorac Surg Clin 2014;24:117-27. [Crossref] [PubMed]
- Lemaire A, Burfeind WR, Toloza E, et al. Outcomes of tracheobronchial stents in patients with malignant airway disease. Ann Thorac Surg 2005;80:434-7; discussion 437-8. [Crossref] [PubMed]
- Saad CP, Murthy S, Krizmanich G, et al. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest 2003;124:1993-9. [Crossref] [PubMed]
- Lin SM, Lin TY, Chou CL, et al. Metallic stent and flexible bronchoscopy without fluoroscopy for acute respiratory failure. Eur Respir J 2008;31:1019-23. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Pitsiou G, et al. Drug Eluting Stents for Malignant Airway Obstruction: A Critical Review of the Literature. J Cancer 2016;7:377-90. [Crossref] [PubMed]
- Chao YK, Liu KS, Wang YC, et al. Biodegradable cisplatin-eluting tracheal stent for malignant airway obstruction: in vivo and in vitro studies. Chest 2013;144:193-9. [Crossref] [PubMed]
- Kiryu T, Hoshi H, Matsui E, et al. Endotracheal/endobronchial metastases : clinicopathologic study with special reference to developmental modes. Chest 2001;119:768-75. [Crossref] [PubMed]
- Park CM, Goo JM, Choi HJ, et al. Endobronchial metastasis from renal cell carcinoma: CT findings in four patients. Eur J Radiol 2004;51:155-9. [Crossref] [PubMed]
- Fournel C, Bertoletti L, Nguyen B, et al. Endobronchial metastases from colorectal cancers: natural history and role of interventional bronchoscopy. Respiration 2009;77:63-9. [Crossref] [PubMed]
- Dalar L, Özdemir C, Sökücü SN, et al. Bronchoscopic palliation to treat endobronchial metastasis of the tracheobronchial tree. Respir Investig 2016;54:116-20. [Crossref] [PubMed]
- Chaussende A, Hermant C, Tazi-Mezalek R, et al. Endobronchial Metastases from Melanoma: A Survival Analysis. Clin Respir J 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Harris K, Puchalski J, Sterman D. Recent Advances in Bronchoscopic Treatment of Peripheral Lung Cancers. Chest 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Kobayashi T, Kaneko M, Sumi M, et al. CT-assisted transbronchial brachytherapy for small peripheral lung cancer. Jpn J Clin Oncol 2000;30:109-12. [Crossref] [PubMed]
- Harms W, Krempien R, Grehn C, et al. Electromagnetically navigated brachytherapy as a new treatment option for peripheral pulmonary tumors. Strahlenther Onkol 2006;182:108-11. [Crossref] [PubMed]
- Harada T, Shirato H, Ogura S, et al. Real-time tumor-tracking radiation therapy for lung carcinoma by the aid of insertion of a gold marker using bronchofiberscopy. Cancer 2002;95:1720-7. [Crossref] [PubMed]


