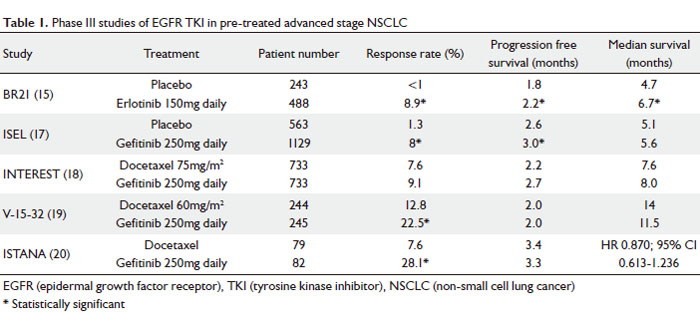
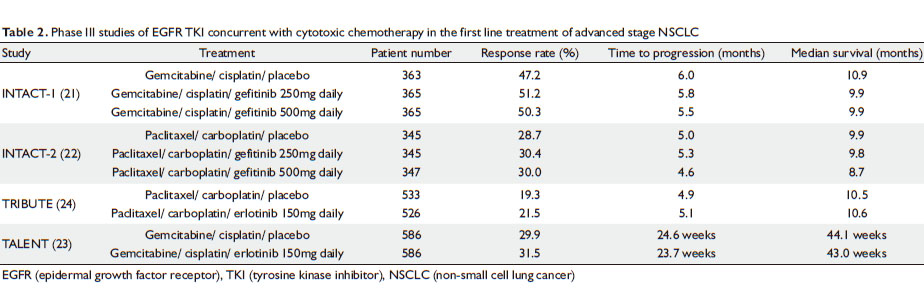
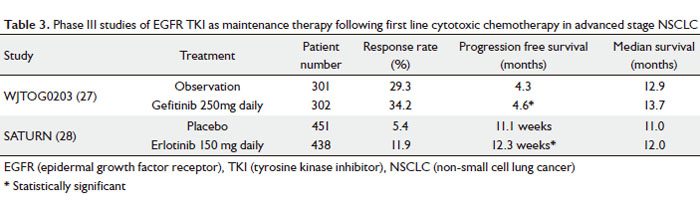
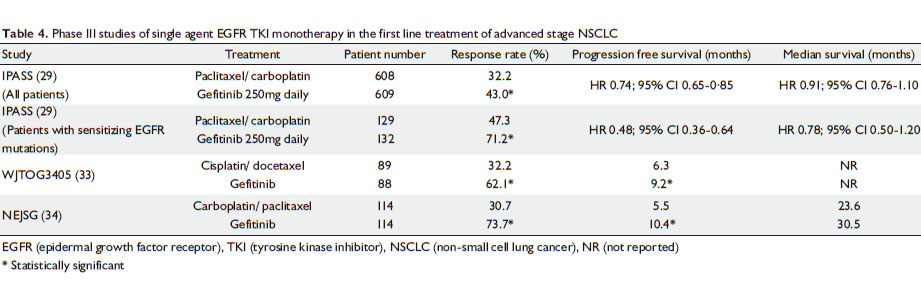
|
References
- NSCLC Meta-Analysis Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non small
cell lung cancer: a systemic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin
Oncol 2008;26:4617-25.[LinkOut]
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. New Engl J Med 2008;358:1160-74.[LinkOut]
- Mendelsohn J, Baselga J. Status of epidermal growth receptor antagonists in the biology and treatment of cancer. J Clin Oncol
2003;21:2787-99. [LinkOut]
- Godin-Heymann N, Bryant I, Rivera M, Ulkus L, Bell D, Riese D, et al. Oncogenic activity of epidermal growth factor receptor
kinase mutant alleles is enhanced by the T790M drug resistance mutation. Cancer Res 2007;67:7319-26. [LinkOut]
- Sharma S, Bell D, Settleman J, Haber D. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81.[LinkOut]
- Ray M, Salgia R, Vokes E. The role of EGFR Inhibition in the treatment of Non-small cell lung Cancer. The Oncologist 2009;14:1116-30.[LinkOut]
- Giaccone G. HER1/EGFR-targeted agents: Predicting the future for patients with unpredictable outcomes to therapy. Ann Oncol
2005;16:538-48. [LinkOut]
- Lynch T, Bell D, Sordella R, Gurubhagavatula S, Okimoto R, Brannigan B, et al. Activating mutations in the epidermal growth
factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [LinkOut]
- Riely G, Pao W, Pham D, Li A, Rizvi N, Venkatraman E, et al. Clinical course of patients with non small cell lung cancer and
epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 2006;12:839-44. [LinkOut]
- Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba I, et al. Clinical and biological features associated with epidermal
growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [LinkOut]
- John T, Liu G, Tsao M. Overview of molecular testing in non-small-cell lung cancer: mutational analysis, gene copy number,
protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene 2009;28:S14-23. [LinkOut]
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard J, et al. Multi-institutional randomized phase II trial of
gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin
Oncol 2003;21:2237-46. Erratum in: J Clin Oncol 2004;22:4811. [LinkOut]
- Kris M, Natale R, Herbst R, Lynch T, Prager D, Belani C, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth
factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 2003;290:2149-58. [LinkOut]
- Cohen M, Williams G, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist
2003;8:303-6. [LinkOut]
- Shepherd F, Perira J, Ciuleanu T, Tan E, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung
cancer. N Engl J Med 2005;353:123-32. [LinkOut]
- Tsao M, Sakurada A, Cutz J, Zhu Q, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer - molecular and clinical predictors
of outcome. N Engl J Med 2005;353:133-44. [LinkOut]
- Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, Von Pawel J, et al. Gefitinib plus best supportive care in
previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled,
multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37. [LinkOut]
- Kim E, Hirsh V, Mok T, Socinski M, Gervais R, Wu Y, et al. Gefitinib vs. docetaxel in previously treated non-small-cell lung
cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18. [LinkOut]
- Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, et al. Phase III study, V-15-32, of gefitinib vs. docetaxel
in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol2008;26:4244-52. [LinkOut]
- Lee D, Park K, Kim J, Lee J, Shin S, Kang J, et al. Randomized Phase III trial of gefitinib vs. docetaxel in non-small cell
lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res 2010;16:1307-14. [LinkOut]
- Giaccone G, Herbst R, Manegold C, Scagliotti G, Rosell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin
in advanced non-small-cell lung cancer: A phase III trial—INTACT 1. J Clin Oncol 2004;22:777-84. [LinkOut]
- Herbst R, Giaccone G, Schiller J, Reck M, Pereira J, Thomas M, et al. Gefitinib in combination with paclitaxel and carboplatin
in advanced non-small-cell lung cancer: A phase III trial—INTACT 2. J Clin Oncol 2004;22:785-94. [LinkOut]
- Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, Rosa F, et al. Phase III study of erlotinib in combination with
cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol
2007;25:1545-52. [LinkOut]
- Herbst R, Prager D, Hermann R, Fehrenbacher L, Johnson B, Sandler A, et al. TRIBUTE: A phase III trial of erlotinib HCl (OSI-774)
combined with carboplatin and paclitaxel chemotherapy in advanced non small cell lung cancer. J Clin Oncol 2005;23:5892-9. [LinkOut]
- Mok T, Wu Y, Yu C, Zhou C, Chen Y, Zhang L, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib
and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2009;27:5080-7. [LinkOut]
- A study of tarceva (erlotinib) or placebo in combination with platinum-based therapy as first line treatment in patients with
advanced or recurrent non-small cell lung cancer [cited 2010 July 13]. Available from: http:// clinicaltrials.gov/ct2/show/NCT00883779.
ClinicalTrials.gov registration number: NCT00883779. [LinkOut]
- Takeda K, Hida T, Sato T, Ando M, Seto T, Satouchi M, et al. Randomized phase III trial of platinum-doublet chemotherapy followed
by gefitinib compared with continued platinum-doublet chemotherapy in Japanese patients with advanced non-small-cell lung
cancer: results of a west Japan thoracic oncology group trial (WJTOG0203). J Clin Oncol 2010;28:753-60. [LinkOut]
- Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E, et al. Erlotinib as maintenance treatment in advanced
non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [LinkOut]
- Mok T, Wu Y, Thongprasert S, Yang C, Chu D, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma.
N Engl J Med 2009;361:947-57. [LinkOut]
- Giaccone G, Gallegos R, Le Chevalier T, Thatcher N, Smit E, Rodriguez JA, et al. Erlotinib for frontline treatment of advanced
non-small cell lung cancer: a phase II study. Clin Cancer Res 2006;12:6049-55. [LinkOut]
- Akerley W, Boucher K, Bentz J, Arbogast K, Walters T. A phase II study of erlotinib as initial treatment for patients with
stage IIIB-IV non-small cell lung cancer. J Thorac Oncol 2009;4:214-9. [LinkOut]
- TORCH: A study of tarceva or chemotherapy for the treatment of advanced non small cell lung cancer [cited 2010 July 7]. Available
from: http://clinicaltrials.gov/ct2/show/NCT00349219. ClinicalTrials.gov registration number: NCT00349219. [LinkOut]
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. West Japan Oncology Group. Gefitinib vs. cisplatin
plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405):
an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [LinkOut]
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung
cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [LinkOut]
- Available online at: http://www.ema.europa.eu/pdfs/human/press/pr/27129209en.pdf. Accessed July 7th, 2010.[LinkOut]
- Lilenbaum R, Axelrod R, Thomas S, Dowlati A, Seigel L, Albert D, et al. Randomized phase II trial of erlotinib or standard
chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol 2008;26:863-9.[LinkOut]
- Goss G, Ferry D, Wierzbicki R, Laurie SA, Thompson J, Biesma B, et al. Randomized phase II study of gefitinib compared with
placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol
2009;27:2253-60. [LinkOut]
- Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, et al. First-line Gefitinib for patients with advanced non-small-cell
lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 2009;27:1394-00. [LinkOut]
- Lee S, Rudd R, Khan I, Upadhyay S, Lewanski CR, Falk S, et al. TOPICAL: Randomized phase III trial of erlotinib compared with
placebo in chemotherapy-naive patients with advanced non-small cell lung cancer (NSCLC) and unsuitable for first-line chemotherapy
[abstract]. J Clin Oncol 2010;28:s7504.
- Herbst R, LoRusso P, Purdom M, Ward D. Dermatologic side effects associated with gefitinib therapy: clinical experience and
management. Clin Lung Cancer 2003;4:366-9. [LinkOut]
- Perez-Soler R, Saltz L. Cutaneous adverse effects with HER1/EGFRtargeted agents: is there a silver lining? J Clin Oncol 2005;23:5235-46.[LinkOut]
- Inoue A, Saijo Y, Maemondo M, Gomi K, Tokue Y, Kimura Y, et al. Severe acute interstitial pneumonia and gefitinib. Lancet
2003;361:137-9. [LinkOut]
- Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, et al. Predictive factors for interstitial lung disease, antitumor
response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 2006;24:2549-56.[LinkOut]
- Hammerman PS, Janne PA, Johnson BE. Resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small
cell lung cancer. Clin Cancer Res 2009;15:7502-9. [LinkOut]
- Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T, et al. Mutations of the epidermal growth factor receptor
gene in lung cancer: Biological and clinical implications. Cancer Res 2004;64:8919-23. [LinkOut]
- Mitsudomi T, Steinberg S, Oie H, Mulshie J, Phelps R, Viallet J, et al. RAS gene mutations in non small cell lung cancers
are associated with shortened survival irrespective of treatment intent. Cancer Res 1991;51:4999-5002. [LinkOut]
- Linardou H, Dahabreh I, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, et al. Assessment of somatic KRAS mutations as
a mechanism associated with resistance to EGFR targeted agents: A systematic review and metaanalysis of studies in advanced
non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol 2008;9:962-72. [LinkOut]
- Greulich H, Chen T, Feng W, Jänne P, Alvarez J, Zappaterra M, et al. Oncogenic transformation by inhibitor-sensitive and -resistant
EGFR mutants. PLoS Med 2005;2:e313. [LinkOut]
- Kobayashi S, Boggon T, Dayaram T, Jänne P, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung
cancer to gefitinib. N Engl J Med 2005;352:786-92. [LinkOut]
- Pao W, Miller VA, Politi KA, Riely G, Somwar R, Zakowski M, et al. Acquired resistance of lung adenocarcinomas to gefitinib
or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [LinkOut]
- Inukai M, Toyooka S, Ito S, Asano H, Ichihara S, Soh J, et al. Presence of epidermal growth factor receptor gene T790M mutation
as a minor clone in non-small cell lung cancer. Cancer Res 2006;66:7854-8. [LinkOut]
- Engelman J, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in
lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43. [LinkOut]
- Yauch R, Januario T, Eberhard D, Cavet G, Zhu W, Fu L, et al. Epithelial vs. mesenchymal phenotype determines in vitro sensitivity
and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res 2005;11:8686-98. [LinkOut]
- Tang J, He Q, Guo R, Chang X. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer
confers poor prognosis. Lung Cancer 2006;51:181-91. [LinkOut]
- Lim W, Zhang W, Miller C, Watters J, Gao F, Viswanathan A, et al. PTEN and phosphorylated AKT expression and prognosis in
early- and late-stage non-small cell lung can cer. Oncol Rep 2007;17:853-7. [LinkOut]
- Morgillo F, Kim W, Kim E, Ciardello F, Hong W, Lee H, et al. Implication of the insulin-like growth factor-IR pathway in the
resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res 2007;13:2795-03. [LinkOut]
- Kwak E, Sordella R, Bell D, Heymann N, Okimoto R, Brannigan B, et al. Irreversible inhibitors of the EGF receptor may circumvent
acquired resistance to gefitinib. Proc Natl Acad Sci U S A 2005;102:7665-70. [LinkOut]
- Li D, Ambrogio L, Shimamura T, Takahashi M, Chirieac LR, Padera R, et al. BIBW2992, an irreversible EGFR/ HER2 inhibitor highly
effective in preclinical lung cancer models. Oncogene 2008;27:4702-11. [LinkOut]
- LUX lung 2 phase II single arm BIBW 2992 in NSCLC with EGFR activating mutations [cited 2010 July 16]. Available from: http://
clinicaltrials.gov/ct2/show/NCT00525148. ClinicalTrials.gov registration number: NCT00525148. [LinkOut]
- BIBW 2992 vs. chemotherapy as first line treatment in NSCLC with EGFR mutation [cited 2010 July 16]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00949650. ClinicalTrials.gov registration number: NCT00949650. [LinkOut]
- Sequist L, Besse B, Lynch T, Miller V, Wong K, Gitlitz B, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase
inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol 2010;28:3076-83. [LinkOut]
- Janne PA, Reckamp K, Koczywas M, Camidge DR, Engelman JA, Khuri F, et al. A phase 2 trial of PF-00299804 (PF299), an oral
irreversible HER tyrosine kinase inhibitor (TKI), in patients (pts) with advanced NSCLC after failure of prior chemotherapy
and erlotinib: preliminary efficacy and safety results. J Thorac Oncol;4:S293-4.Abstr A3.1.
- Yoshimura N, Kudoh S, Kimura T, Mitsuoka S, Matsuura K, Hirata K, et al. EKB-569, a new irreversible epidermal growth factor
receptor tyrosine kinase inhibitor, with clinical activity in patients with non-small cell lung cancer with acquired resistance
to gefitinib. Lung Cancer 2006;51:363-8. [LinkOut]
- De Boer R, Arrieta Ó, Gottfried M, Blackhall F, Yang C, Langmuir P, et al. Vandetanib plus pemetrexed vs. pemetrexed as second-line
therapy in patients with advanced non small cell lung cancer (NSCLC): A randomized, double-blind phase III trial (ZEAL) [abstract].
J Clin Oncol 2009;27:8010.
- Natale R, Thongprasert S, Greco F, Greco A, Thomas M, Tsai C, et al. Vandetanib vs. erlotinib in patients with advanced non-small
cell lung cancer (NSCLC) after failure of at least one prior cytotoxic chemotherapy: A randomized, double-blind phase III
trial (ZEST) [abstract]. J Clin Oncol 2009;27:8009.
- Herbst R , Sun Y, Korfee S, Eberhadt W, Geermonpre P, Saijo N, et al. Vandetanib plus docetaxel vs. docetaxel as second-line
treatment for patients with advanced non-small cell lung cancer (NSCLC): A randomized, double-blind phase III trial (ZODIAC).
Lancet Oncol 2010;11:619-26. [LinkOut]
- Bahleda R, Soria J, Harbison C, Park J, Felip E, Hanna N, et al. Tumor regression and pharmacodynamic (PD) biomarker validation
in non-small cell lung cancer (NSCLC) patients treated with the ErbB/VEGFR inhibitor BMS-690514 [abstract]. J Clin Oncol 2009;27:8098.
Cite this article as: Voon PJ, Cho BC, Yeo WL, Soo RA. The role of epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of advanced stage non-small cell lung cancer. J Thorac Dis 2010;2(3):144-153. doi: 10.3978/j.issn.2072-1439.2010.02.03.6
|