Relationship between polycythemia and in-hospital mortality in chronic obstructive pulmonary disease patients with low-risk pulmonary embolism
Introduction
The morbidity and mortality associated with chronic obstructive pulmonary disease (COPD) is rising and is projected to become the third leading worldwide cause of death by 2020 (1,2). COPD is associated with an increased risk of deep venous thrombosis (DVT) and pulmonary embolism (PE), particularly during exacerbations, and with an increased risk of mortality after PE (3,4). In COPD patients with PE, the 3-month mortality rate is twice compared to the reported 15% to 18% in general population (5); however, risk factors for mortality due to PE in patients with COPD are not well known, even in the COPD patients classified as low risk for PE. Endothelial dysfunction, coexistent pulmonary hypertension (PH), and in general low cardiorespiratory reserve, have been invoked as potential risk factors for mortality (6). Another important physiologic abnormality in COPD is the presence of polycythemia, a potential contributor to the development of PH and pulmonary endothelial dysfunction (7-9). We hypothesize that polycythemia is an independent contributing factor to mortality due to PE in patients with COPD, while controlling for other factors associated with PE related mortality. We tested this hypothesis using clinical data from a multi-center retrospective cohort study in a large population area of Sichuan province in southwestern China.
Methods
Specimens and subject populations
This multicenter, retrospective and administrative data based study was conducted at two largest tertiary university hospitals in Sichuan province serving much of southwest of China: West China Hospital of Sichuan University and Sichuan Provincial People’s Hospital with total 7,777 in-patient beds.
Methods
We included all consecutive patients who were admitted in the two hospitals with a diagnosis of PE and COPD during the period of October, 2005 and October, 2015. PE was diagnosed by computerized tomography angiography (CTA). We used clinical and demographic variables to calculate a PESI score for all PE patients. PESI score calculations were performed using the standard PESI definition: low risk (class I and II, PESI score ≤85), moderate to high risk (class III–V, PESI score >85) (10,11). Subjects in risk classes III–V on the basis of the PESI score (>85) were excluded as clinical guidelines do not consider such patients in low risk category. A diagnosis of COPD was based on the Global Strategy for Diagnosis, Management, and Prevention of COPD (GOLD 2011) criteria of a post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ≤70% (2). Severity of COPD (COPD stage) was classified according to the Medical Research Council (mMRC) dyspnea score, FEV1 predicted value ratio (FEV1% predicted) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011 staging system (2). Patients with incidental findings of PE in CTA examinations (PE was diagnosed by CTA ordered incidentally within 24 hours of hospitalization) were also included.
Pulmonary CTA was performed on a 64-slice multi-detector CT scanner (Definition Flash, 120 kvp, Siemens, Erlangen, Germany) within 24 hours of admission. The diagnosis of PE was made when an intraluminal filling defect surrounded by a rim of intravascular contrast or total occlusion of the pulmonary arterial lumen was detected at any level of the pulmonary arteries (12). The anatomic location of the emboli was noted in the clinical record.
Spirometry was measured within the first 3 days of admission by trained technicians with MS PFT (VIASYS, US) spirometer, and was performed according to standards set by the American Thoracic Society/European Respiratory Society guidelines (13).
Exclusion criteria were patients in risk classes III-V on the basis of the PESI score (>85); primary polycythemia (i.e., polycythemia vera), prior diagnosis of PE already under treatment, known malignancies, paralysis & immobility (bed rest >48 h) (Figure 1).
Outcomes
The primary outcome was all-cause in-hospital mortality. To ascertain this, all subjects were followed until discharge and final disposition was confirmed with clinical records. To ascertain this, all subjects were reviewed and data extracted following a standard protocol by four researchers (L Guo, Z Xie, H Jiang and L Gao). Secondary outcomes included length of stay in hospital and frequency of noninvasive and invasive mechanical ventilation during the hospital stay.
Exposure
Polycythemia was defined as a hemoglobin (Hb) level ≥180 g/L in men, and ≥150 g/L in women. All blood analyses were performed in agreement with the “Recommendation on sampling, transport, and storage for the determination of the concentration of ionized calcium in whole blood, plasma, and serum” quality standards (14). Systematically, we recorded the results of blood samples drawn within 24 h of admission in every patient.
Covariates
Baseline characteristics included demographic and clinical data, including smoking history and COPD co-morbidities recorded with international classification of diseases (ICD)-10 codes.
Arterial blood gas (ABG) analysis was performed with a GEM Premier™ 3000 blood gas/electrolyte analyzer (GEM Premier 3000, USA). Laboratory biochemical variables were analyzed by automatic biochemical analyzer according to the same quality standards between the two hospitals (15). All laboratory measures were performed at admission. All electrocardiograms (ECGs) abnormal evidences were tracked for each patient by systematically searching for diagnosis with ICD-10 code and medical records.
Deep veins of the lower extremities and echocardiography detected by Doppler ultrasonography were collected simultaneously for every patient within 5 days of hospitalization by experienced sonographers with Color Doppler Ultrasound (iU22 Ultrasound System; PHILIPS, Netherlands and iE33 xMATRIX Echocardiography System, PHILIPS, Netherland, respectively). All the data and diagnoses were acquired following the standard guideline (16,17).
Estimation of systolic pulmonary arterial pressure (sPAP): the echocardiographic study of a patient with a suspected PH in our study was based on the peak velocity of the tricuspid regurgitation jet (TRV) by continuous-wave (CW) Doppler. We used the simplified Bernoulli equation to assess the TRV [peak pressure; sPAP = 4 × (TRV)2 + RA v-wave ≈ 4 × (TRV)2 + mean RAP, where RA is right atrium and RAP is right atrial pressure] describes the relationship of TR and RVSP as a surrogate of sPAP in the absence of RV outflow tract (RVOT) obstruction, pulmonary valve stenosis, or PA stenosis (16). PH was diagnosed by echocardiography examination (TRV >2.8 m/s and sPAP >36 mmHg) (18). According to international standards of PH classification, patients were divided into three groups: normal group (sPAP <36 mmHg) the mild to moderate group (36≤ sPAP <70 mmHg) and the severe group (sPAP ≥70 mmHg) (19).
The patient was considered obese if the body mass index (BMI) was ≥30 kg/m2 (20). Diagnosis of hypertension, diabetes mellitus, coronary artery disease, known heart failure or known cerebral infarction was based on medical records with compatible ICD-10 codes (21-25). Smoking history in the study included current or former smoking, and pack-years smoked.
Ethical considerations
All procedures were approved by the Research and Ethics Committee of Medicine in both hospitals (number/ID of the approval: 2009-42).
Statistical analyses
Baseline characteristics are reported as the mean and standard deviation for continuous variables and as percentages for categorical variables. Differences in baseline characteristics between the two groups, based on the presence of polycythemia, of COPD patients were determined using the student “t-test” for continuous variable and the chi-square test for categorical variables. A P value <0.05 in 2-tailed tests was considered statistical significant. We analyzed the impact of polycythemia in mortality using multivariate models additionally adjusted for other variables known to influence COPD outcomes (gender, age, hospitalization/year, sPAP and co-morbidities) and secondary polycythemia (FEV1, pO2), based on their bivariate association with mortality. All the data was analyzed using the Statistical Package for the Social Sciences 16.0 packet program (SPSS Inc., Chicago, IL, USA).
Results
Demographics, clinical characteristics of the study population
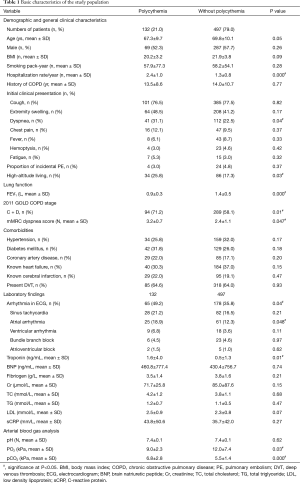
We retrospectively enrolled a total of 1,043 consecutive patients with COPD and PE who were admitted to hospital during the study period. Among them, 414 met the exclusion criteria [cause (number)]: patients in risk classes III–V on the basis of the PESI score [161]; primary polycythemia [5]; prior diagnosis of PE already under treatment [17]; known malignancies [153]; paralysis & immobilized more than 48 hrs [78]. Thus, 629 patients with COPD and low-risk PE fulfilled the inclusion criteria and were finally included [60.5% males; age, 69.6±10.1 years (mean ± SD); FEV1% predicted, 57.9%±19.0% (mean ± SD)], in whom there were 132 with COPD and secondary polycythemia (21.0%) and 497 with COPD but no secondary polycythemia (79.0%) (Figure 1). Medical records showed 4 patients (3.0%) in polycythemia group and 24 (4.8%) patients in the group without polycythemia were sent to hospital with an initial diagnosis of acute exacerbation of COPD and were diagnosed incidentally of PE with the same complaints during their extended stay in hospital. There were no significant differences in age, gender and BMI (P=0.05, P=0.26 and P=0.09, respectively) as well as smoking pack-year (P=0.28) and history of COPD (P=0.77). A history of annual COPD-related hospitalizations was more frequent (2.4±1.0 vs. 1.3±0.8, P=0.000) among COPD with polycythemia. Patients with COPD were less likely to have symptomatic PE. However, COPD patients with polycythemia were more likely to report dyspnea compared to those without polycythemia. A lower FEV1 level (0.93±0.3 vs. 1.4±0.5, P=0.000) and higher mMRC dyspnea scores (3.2±0.7 vs. 2.4±1.1, P=0.047) were observed in COPD cohort with polycythemia. Accordingly, more patients with polycythemia belonged to 2011 GOLD COPD stages C and D (more symptoms and high risks) (71.2% vs. 58.1%, P=0.01). Our results indicated a higher proportion of COPD patients with polycythemia led high-altitude living than those without polycythemia (25.8% vs. 17.3%, P=0.03). Patients with COPD were less likely to have symptomatic PE. However, COPD patients with polycythemia were more likely to report dyspnea compared with those without polycythemia. There were no difference in the frequency of COPD co-morbidities (including hypertension, diabetes mellitus, known heart failure, known cerebral infarction and DVT) observed between the groups with or without polycythemia (all P>0.05).
There was no difference in the initiation of anticoagulant therapy in both groups (1.6±2.2 and 1.8±2.1, respectively). The anticoagulant therapy wasn’t initiated immediately at admission in cases with the high risk of bleeding with renal or liver dysfunction (53 patients), uncontrolled hypertension (44 patients), incidental findings of PE (28 patients) or failure to obtain consent from the patient or designated relative with power of attorney (31 patients). Anticoagulant therapy was initiated when their situation permitted.
The laboratory results demonstrated a higher proportion of arrhythmia on ECG (49.2% vs. 35.8%, P=0.04) and a higher level of troponin (1.6±4.0 vs. 0.5±1.3, P=0.01) among the COPD patients with polycythemia compared to the comparison cohort. The electrocardiographic abnormalities investigated were: (I) sinus tachycardia (>100 beats/min); (II) atrial arrhythmia; (III) ventricular arrhythmia; (IV) bundle branch block (complete or incomplete); and (V) atrioventricular block (multiple ECG abnormalities in some patients). ECG data showed that atrial arrhythmia was significantly more frequent in polycythemia group. The other laboratory findings, as levels of N-terminal pro-brain natriuretic peptide (BNP), fibrinogen, creatinine (Cr), total cholesterol (TC), total triglyceride (TG), low density lipoprotein (LDL) and C-reactive protein (sCRP) were similar in both groups (all P>0.05). Significantly lower levels of PaO2 (9.0±2.3 vs. 12.0±7.4, P=0.03) and blood O2 saturation (SpO2) (88.1±5.9 vs. 93.4±7.2, P=0.03) as well as higher level of PaCO2 (6.8±2.8 vs. 5.5±1.4, P=0.000) on ABG analyses were observed in polycythemia cohort than those in the other group (Table 1).
Full table
Imaging findings
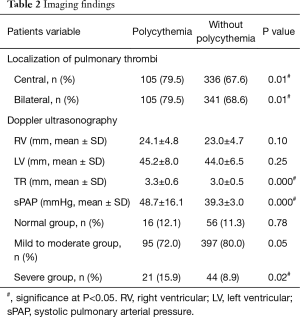
Central PE (i.e., main and segmental pulmonary arteries) on pulmonary CTA was more common (79.5% vs. 67.6%, P=0.01) while bilateral PE were more prevalent (79.5% vs. 68.6%, P=0.01) among those with polycythemia. In addition, higher velocity of TR (3.3±0.6 vs. 3.0±0.5, P=0.000) and higher level of sPAP (48.7±16.1 vs. 39.3±3.0, P=0.000) were also observed via Doppler ultrasonography in the same population. Non-significant differences of the diameters of right ventricular (RV) and left ventricular (LV) were found between groups (P>0.05).
There were 16 (12.1%), 95 (72.0%) and 21 (15.9%) patients classified in the normal, mild to moderate and severe group of PH respectively among patients with polycythemia. Correspondingly, there were 56 (11.3%), 397 (80.0%) and 44 (8.9%) patients classified in the normal, mild to moderate and severe group of PH respectively among patients without polycythemia. More patients with severe PH were in the polycythemia group (P=0.02) (Table 2).
Full table
Hospital treatment and outcomes by polycythemia group
There was no significant difference in initiation of anticoagulant therapy after admission between groups (1.6±2.2 vs. 1.8±2.1, P=0.42). COPD patients with polycythemia had a longer hospital stay (15.3±10.1 vs. 9.7±9.1, P=0.001), a higher mechanical ventilation rate (noninvasive and invasive, 53.8% vs. 43.9%, P=0.04 and 33.3% vs. 22.9%, P=0.01, respectively), and a higher in-hospital mortality (12.1% vs. 6.6%, P=0.04) (Table 3).

Full table
The receiver-operating characteristic (ROC) curve to evaluate Hb level for mortality
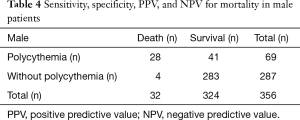
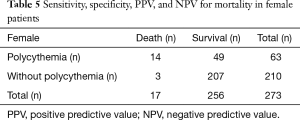
We performed the ROC curve to evaluate Hb level for mortality (male: AUC 0.70, 95% CI, 0.62–0.79; female: AUC 0.74, 95% CI, 0.68–0.79). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for mortality were 87.5% (28/32), 87.3% (283/324), 40.6% (28/69), 98.6% (283/287) in male and 82.4% (14/17), 80.9% (207/256), 22.2% (14/63), 98.6% (207/210) in female respectively (Tables 4,5).
Full table
Full table
Associations between polycythemia and mortality
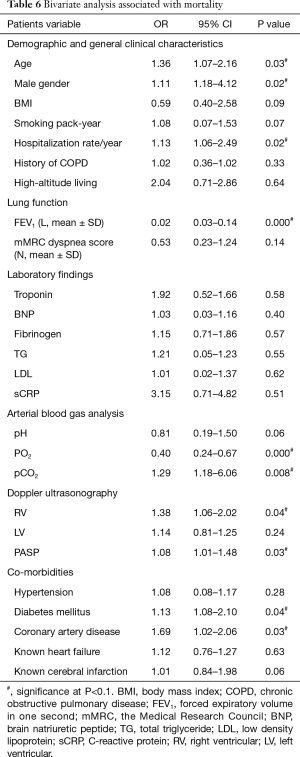
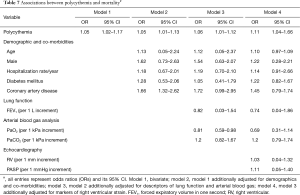
Polycythemia was associated with mortality as determined on bivariate analysis (OR 1.05, 95% CI, 1.02–1.17). This association was maintained in models additionally adjusted for demographics (age, gender and hospitalization/year) and co-morbidities (diabetes mellitus and coronary artery disease) (OR 1.05, 95% CI, 1.01–1.13), descriptors of lung function and ABG (FEV1, PaO2) (OR 1.06, 95% CI, 1.01–1.12), and markers of RV strain (RV, sPAP) (OR 1.11, 95% CI, 1.04–1.66) (Tables 6,7).
Full table
Full table
Discussion
A recent large meta-analysis confirmed the role of the PESI score of I–II in the identification of PE patients at a low risk of mortality (26). However, some of those patients with COPD may still have an elevated risk of clinical deterioration and in-hospital death. Thus, a careful stratification of patients with acute PE seems to be particularly important for clinicians to guide the initial management and to protect patients against the hazard to be treated with an unacceptable level of risk, or to undergo unnecessary and potentially dangerous treatment.
Although data to support a key role for secondary polycythemia with increased the risk of PE is scant, we observed that PE with polycythemia was associated with increased all-cause in-hospital mortality rate in this multicenter, retrospective cohort study. Our results are similar with Weber et al. who observed increased mortality due to the presence of polycythemia in 1913 (27).
Medical records showed 4 patients (3.0%) in polycythemia group and 24 (4.8%) patients in the other group were sent to hospital with an initial diagnosis of acute exacerbation of COPD and an incidental diagnosis of PE and with identical complaints during their extended stay in hospital. It was remarkable to observe that no-specific symptoms of PE were described in most of the cases, thus supporting the concept that clinical suspicion of PE in acute exacerbation of COPD is particularly difficult, for clinical symptoms of COPD may mimic PE symptoms. Most of patients with PE were hospitalized for an episode of exacerbation of COPD and it always took 2 or 3 days to make a definite diagnosis of PE.
In this study, we also observed that polycythemia in COPD patients was associated with more frequent re-hospitalization per year, more frequent rate of arrhythmia, a longer hospital stay and increased rate of mechanical ventilation (including noninvasive), compared with COPD patients without polycythemia. Overall, polycythemia could be an indicator of worse outcome in COPD patients with PE which warrants lower threshold for the clinical suspicion of PE in this group.
Lippmann and Fein (28) suggested that the diagnosis of PE in patients with COPD should be suspected in patients with precipitous worsening of their dyspnea that is unresponsive to bronchodilator therapy. The diagnosis is supported by a reduction in the PaCO2 in a previously hypercapnic patient (28). In our retrospective study, it was uncertain that patients were previously hyoxemia and hypercapnic or not. However, we found significantly lower PaO2 levels as well as a higher PaCO2 levels on ABG analyses in COPD patients with polycythemia. There is good evidence to suggest that hypoxemia has a strong association with advanced COPD. Furthermore, it now seems clear that tissue hypoxia is a key player in many of the maladaptive processes and extra-pulmonary co-morbidities that characterize COPD (29,30). On the other hand, COPD has long been recognized as an important cause of secondary polycythemia due to hypoxemia. Several studies postulate hypercapnia as an independent predictor for survival and in-hospital deaths in the COPD patients (31-33). The finding of an elevation in the PaCO2 in the presence of PE has been reported (34). It may reflect an inability to further increase minute ventilation in the face of a sudden increase in dead space ventilation imposed by the embolus (35). The finding of an elevation in the PaCO2 in the presence of PE has been reported (34). It may reflect an inability to further increase minute ventilation in the face of a sudden increase in dead space ventilation imposed by the embolus (35). Polycythemia, which increases blood viscosity, may increase hypoxemia and hypercapnic risks in COPD patients. Depending on the PaO2 and PaCO2 levels at admission, these physiological differences may affect survival in the COPD patient group being treated with mechanical ventilation due to respiratory failure, indicating that polycythemia can attenuate lung function via various mechanisms causing worse prognosis.
The most striking difference in our study was that higher proportion of patients with polycythemia were in advanced COPD stage (2011 GOLD COPD stages C and D) with more severe symptomatic dyspnea, higher mMRC dyspnea score) and lower FEV1 level, which suggests that patients with specific high Hb response to hypoxia and deteriorated lung function may be associated with increased mortality and poor quality of life compared with the general COPD population (36,37). This individualized response to infection or another exacerbation triggers may identify patients more likely to have a pro-inflammatory response to subsequent exacerbations and explain the observed increase in in-hospital mortality, which is significant than previously recognized.
Our data demonstrated centrally and bilaterally located emboli were more common (79.8% and 81.8%, respectively) in COPD patients with polycythemia in our study, which explains the poor outcomes, as the mortality rate remains high in patients with centrally located PE (38).
Although mild to moderate level of PH is a common consequence of COPD, especially in the COPD patients with PE (39,40), we observed more severe PH in the population with polycythemia. Our data supports previous studies which showed the contribution of polycythemia to PH (41,42). One possibility is that the severity of pulmonary vascular remodeling may increase the effect of Hb, thereby worsening PH when pulmonary vascular compliance is decreased by vascular remodeling (41,42). Moreover, a previous study demonstrated that repetitive hemodilution in patients with COPD can improve PH by reducing blood viscosity (43). Increased viscosity is therefore another contributing factor in secondary PH in COPD patients (43). Since PH has an independent prognostic impact on survival (44), the prognosis is poorer in the COPD patients with polycythemia. However, we didn’t following up these patients, so the sPAP in our study could probably reflected the situation at acute period.
ECG features showed sinus tachycardia is the most common ECG abnormality in both groups. Moreover, arrhythmia especially atrial arrhythmia was significantly frequent in polycythemia group. It has previously been observed in previous studies that atrial arrhythmia could be an independent predictor of increased mortality in patients with PE (45). On the other hand, COPD is independently associated with an increased burden of cardiovascular disease, and specific ECG abnormalities and cardiac arrhythmias seem to have a significant effect on cardiovascular prognosis of COPD patients (46). Screening for arrhythmia may still be of importance due to its clinical impact on a poor prognosis in this population.
In this study, we demonstrated that COPD patients living in high-altitude area tend to contribute to the development of secondary polycythemia. Red blood cell counts and Hb concentrations increase to maintain oxygen transport in the hypobaric environment at high altitudes which are regarded risk factors for thrombosis as well (47). Early detection and treatment of PE in this population should help decrease the morbidity and mortality significantly.
Co-morbid factors associated with PE in the study including age, gender, obesity, smoking, hyperlipidemia, hypertension, diabetes mellitus, coronary artery disease, known heart failure, known cerebral infarction, and DVT were recorded at the baseline as potential confounding factors (48-51). However, our study did not reveal significant relationship between these confounders with in-hospital mortality.
It must be emphasized that multivariate logistic regression analysis in our study showed that polycythemia is an independent risk factor related to mortality in COPD patients with PE. It is also suggested that decreasing PaO2 and FEV1 as well as increasing PaCO2, RV and PH may have a significant effect on the poor outcome. There is a trend towards earlier detection and initiating earlier therapeutic interventions in COPD patients to prevent the development of polycythemia. It is also important to note the importance of monitoring lung function in the management of this cohort. Therefore, we aimed to provide an opportunity to identify high risk patients with poor prognosis in COPD population.
Our study had following limitations. This was a retrospective and administrative data based study, therefore, selection bias could be present. The COPD patients with PE were hospitalized and were mostly in critical condition and could not tolerate right heart catheterization. As pulmonary hyperinflation is frequently seen in COPD patients, the likelihood of estimating PASP would be lower in patients with marked pulmonary hyperinflation via echocardiography. In addition, the prevalence and the characteristics of COPD patients with polycythemia have significant geographical diversity. A study with a larger cohort from geographical areas of different altitudes should be undertaken in the future.
In conclusion, polycythemia is an independent risk factor for all-cause in-hospital mortality in COPD patients with PE at low risk and may require a lower threshold for evaluating these patients for PE as they present with severe hypoxia, higher PH, worse pulmonary functions and higher frequency of centrally located PE.
Acknowledgements
Funding: The study protocol was mentored by Methods in Epidemiologic, Clinical and Operations Research (MECRO) training, which sponsored by American Thoracic Society (ATS). Especially appreciate to our facilitator: Prof. Jeffrey L. CURTIS (Pulmonary& Critical Care Medicine Division, University of Michigan Health System, Ann Arbor, MI 48109, USA). The research was supported by a grant-in-aid for “Establishment the clinical database and diagnosis system of PH in local area” (No. 090442) from Health Department of Sichuan Province, China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Research and Ethics Committee of Medicine in both hospitals (No. 2009-42).
References
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [Crossref] [PubMed]
- Wen FQ, He B. Interpretation of Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (GOLD) (revised 2011). Zhonghua Yi Xue Za Zhi 2012;92:939-40. [PubMed]
- Laporte S, Mismetti P, Décousus H, et al. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation 2008;117:1711-6. [Crossref] [PubMed]
- Poulsen SH, Noer I, Møller JE, et al. Clinical outcome of patients with suspected pulmonary embolism. A follow-up study of 588 consecutive patients. J Intern Med 2001;250:137-43. [Crossref] [PubMed]
- Rizkallah J, Man SF, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest 2009;135:786-93. [Crossref] [PubMed]
- Hopkins N, McLoughlin P. The structural basis of pulmonary hypertension in chronic lung disease: remodelling, rarefaction or angiogenesis? J Anat 2002;201:335-48. [Crossref] [PubMed]
- Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis 2011;6:199-208. [PubMed]
- Pierson DJ. Pathophysiology and clinical effects of chronic hypoxia. Respir Care 2000;45:39-51; discussion 51-3. [PubMed]
- Vaidyula VR, Criner GJ, Grabianowski C, et al. Circulating tissue factor procoagulant activity is elevated in stable moderate to severe chronic obstructive pulmonary disease. Thromb Res 2009;124:259-61. [Crossref] [PubMed]
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315-52. [Crossref] [PubMed]
- Konstantinides SV, Torbicki A, Agnelli G, et al. Corrigendum to: 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2015;36:2642. [Crossref] [PubMed]
- Remy-Jardin M, Pistolesi M, Goodman LR, et al. Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology 2007;245:315-29. [Crossref] [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Boink AB, Buckley BM, Christiansen TF, et al. Recommendation on sampling, transport, and storage for the determination of the concentration of ionized calcium in whole blood, plasma, and serum. IFC Scientific Division, Working Group on Ion-Selective Electrodes (WGSE). J Int Fed Clin Chem 1992;4:147-52. [PubMed]
- Burnett RW, Covington AK, Fogh-Andersen N, et al. International Federation of Clinical Chemistry (IFCC). Scientific Division. Committee on pH, Blood Gases and Electrolytes. Approved IFCC recommendations on whole blood sampling, transport and storage for simultaneous determination of pH, blood gases and electrolytes. Eur J Clin Chem Clin Biochem 1995;33:247-53. [PubMed]
- Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003;108:1146-62. [Crossref] [PubMed]
- Guideline developed in collaboration with the American College of Radiology; Society of Pediatric Radiology; Society of Radiologists in Ultrasound. AIUM Practice Guideline for the Performance of Peripheral Venous Ultrasound Examinations. J Ultrasound Med 2015;34:1-9. [Crossref]
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573-619. [Crossref] [PubMed]
- Sciomer S, Badagliacca R, Fedele F. Pulmonary hypertension: echocardiographic assessment. Ital Heart J 2005;6:840-5. [PubMed]
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr 1998;68:899-917. [PubMed]
- Update on current care guideline: hypertension. Duodecim 2014;130:2366-8. [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37 Suppl 1:S81-90. [Crossref] [PubMed]
- Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002;106:388-91. [Crossref] [PubMed]
- Chinese Society of Cardiology of Chinese Medical Association; Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for the diagnosis and treatment of heart failure 2014. Zhonghua Xin Xue Guan Bing Za Zhi 2014;42:98-122. [PubMed]
- Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:3020-35. [Crossref] [PubMed]
- Squizzato A, Donadini MP, Galli L, et al. Prognostic clinical prediction rules to identify a low-risk pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost 2012;10:1276-90. [Crossref] [PubMed]
- Weber FP. The Prognostic Significance of Secondary Polycythæmia in Cardio-pulmonary Cases. Proc R Soc Med 1913;6:83-98.
- Lippmann M, Fein A. Pulmonary embolism in the patient with chronic obstructive pulmonary disease. A diagnostic dilemma. Chest 1981;79:39-42. [Crossref] [PubMed]
- Maekura R, Hiraga T, Miki K, et al. Personalized pulmonary rehabilitation and occupational therapy based on cardiopulmonary exercise testing for patients with advanced chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015;10:1787-800. [Crossref] [PubMed]
- Yoshimura K, Maekura R, Hiraga T, et al. Identification of three exercise-induced mortality risk factors in patients with COPD. COPD 2014;11:615-26. [Crossref] [PubMed]
- Oga T, Taniguchi H, Kita H, et al. Analysis of the relationship between health status and mortality in hypercapnic patients with noninvasive ventilation. Clin Respir J 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Ahmadi Z, Bornefalk-Hermansson A, Franklin KA, et al. Hypo- and hypercapnia predict mortality in oxygen-dependent chronic obstructive pulmonary disease: a population-based prospective study. Respir Res 2014;15:30. [Crossref] [PubMed]
- Soltani A, Reid D, Wills K, et al. Prospective outcomes in patients with acute exacerbations of chronic obstructive pulmonary disease presenting to hospital: a generalisable clinical audit. Intern Med J 2015;45:925-33. [Crossref] [PubMed]
- Lesser BA, Leeper KV Jr, Stein PD, et al. The diagnosis of acute pulmonary embolism in patients with chronic obstructive pulmonary disease. Chest 1992;102:17-22. [Crossref] [PubMed]
- D'Alonzo GE, Bower JS, DeHart P, et al. The mechanisms of abnormal gas exchange in acute massive pulmonary embolism. Am Rev Respir Dis 1983;128:170-2. [Crossref] [PubMed]
- Ryynänen OP, Soini EJ, Lindqvist A, et al. Bayesian predictors of very poor health related quality of life and mortality in patients with COPD. BMC Med Inform Decis Mak 2013;13:34. [Crossref] [PubMed]
- Liu SF, Tseng CW, Tu ML, et al. The clinical COPD questionnaire correlated with BODE index-A cross-sectional study. ScientificWorldJournal 2012;2012:361535.
- Klok FA, Djurabi RK, Nijkeuter M, et al. High D-dimer level is associated with increased 15-d and 3 months mortality through a more central localization of pulmonary emboli and serious comorbidity. Br J Haematol 2008;140:218-22. [PubMed]
- Barberà JA, Blanco I. Pulmonary hypertension in patients with chronic obstructive pulmonary disease: advances in pathophysiology and management. Drugs 2009;69:1153-71. [Crossref] [PubMed]
- Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:189-94. [Crossref] [PubMed]
- Barer GR, Bee D, Wach RA. Contribution of polycythaemia to pulmonary hypertension in simulated high altitude in rats. J Physiol 1983;336:27-38. [Crossref] [PubMed]
- Nakamura A, Kasamatsu N, Hashizume I, et al. Effects of hemoglobin on pulmonary arterial pressure and pulmonary vascular resistance in patients with chronic emphysema. Respiration 2000;67:502-6. [Crossref] [PubMed]
- Borst MM, Leschke M, König U, et al. Repetitive hemodilution in chronic obstructive pulmonary disease and pulmonary hypertension: effects on pulmonary hemodynamics, gas exchange, and exercise capacity. Respiration 1999;66:225-32. [Crossref] [PubMed]
- Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 2008;32:1371-85. [Crossref] [PubMed]
- Escobar C, Jiménez D, Martí D, et al. Prognostic value of electrocardiographic findings in hemodynamically stable patients with acute symptomatic pulmonary embolism. Rev Esp Cardiol 2008;61:244-50. [Crossref] [PubMed]
- Goudis CA, Konstantinidis AK, Ntalas IV, et al. Electrocardiographic abnormalities and cardiac arrhythmias in chronic obstructive pulmonary disease. Int J Cardiol 2015;199:264-73. [Crossref] [PubMed]
- Rathi K, Uppal V, Bewal N, et al. D-dimer in the diagnostic workup of suspected pulmonary thrombo-embolism at high altitude. Med J Armed Forces India 2012;68:142-4. [Crossref] [PubMed]
- Nadeem O, Gui J, Ornstein DL. Prevalence of venous thromboembolism in patients with secondary polycythemia. Clin Appl Thromb Hemost 2013;19:363-6. [Crossref] [PubMed]
- Lubarsky DA, Gallagher CJ, Berend JL. Secondary polycythemia does not increase the risk of perioperative hemorrhagic or thrombotic complications. J Clin Anesth 1991;3:99-103. [Crossref] [PubMed]
- Ristić L, Rančić M, Radović M, et al. Pulmonary embolism in chronic hypoxemic patients with and without secondary polycythemia--analysis of risk factors in prospective clinical study. Med Glas (Zenica) 2013;10:258-65. [PubMed]
- Bhatt VR. Secondary polycythemia and the risk of venous thromboembolism. J Clin Med Res 2014;6:395-7. [PubMed]



