Left thoracotomy for middle or lower thoracic esophageal carcinoma: still Sweet enough?
Introduction
Esophageal carcinoma is a frequently-occurring malignant tumors, and its global morbidity and mortality rates rank as the 8th and 6th of all cancers, respectively (1). Despite the extensive application of multimodal therapy, the 5-year overall survival (OS) rate remains low. At present, surgery is still the main strategy for locally-advanced esophageal carcinoma (2), although controversy persists regarding the surgical approach for middle or lower thoracic esophageal squamous cell carcinoma (OSCC-MLT). The transthoracic or transhiatal approach for adenocarcinoma is under dispute in Western countries (3-5); however, in China, the common debate is between the Sweet procedure and the Ivor-Lewis (IL) procedure for esophageal squamous cell carcinoma (6-9).
The Sweet procedure, which is the conventional route for resection of esophageal carcinoma, outperforms the IL procedure in many ways, such as being a simpler operation with a shorter operative time and an increase in tolerance from patients. However, this procedure is mainly debated mainly because of the limited extent of lymphadenectomy, especially for the dissection of the right upper mediastinal lymph nodes (RUM-LND). A meta-analysis shows that the Sweet procedure is inferior to the IL procedure in lymph node dissection (6). However, after comparing 748 Sweet-treated and 167 IL-treated patients with esophageal carcinoma in 2015, Ma et al. demonstrated no significant difference in the number of lymph node dissections or total number of cases with lymph node metastases (7).
At present, there is no consensus on the extent of lymphadenectomy for esophageal carcinoma. Some studies indicate that more extensive lymph node dissection might not increase the 5-year OS rate but that is only improves N staging (10,11), which is in opposition to current clinical guidelines. Similarly, right upper mediastinal lymph node metastasis (RUM-LNM) predicts a poor prognosis for esophageal carcinoma patients but is not associated with the 5-year OS (12). However, by identifying 4627 esophageal cancer patients undergoing the operation from the Worldwide Esophageal Cancer Collaboration database, Rizk et al. demonstrated that a greater extent of lymph node dissection is associated with better survival, except for those staging as TisN0M0 and G1 pN0M0 (13).
To explore the optimal surgical procedure that could reduce surgical trauma and improve outcomes in OSCC-MLT patients, experienced surgeons in our department started to perform a modified Sweet (MS) procedure: the traditional Sweet procedure with RUM-LND. The MS procedure has been rarely reported. The MS procedure has the merits of the Sweet procedure and addresses its limitations in the lymph node dissection; therefore, the MS procedure is at least equivalent to the IL procedure. In this study, we retrospectively compared the MS procedure with the IL procedure in terms of lymphadenectomy, postoperative complications, and long-term survival. The feasibility and effectiveness of the MS procedure in the treatment of OSCC-MLT were evaluated.
Methods
Patients
This retrospective study was approved by the Ethics Committee at the West China Hospital of Sichuan University (No. 201649). By searching the esophageal carcinoma database, we identified the records of all OSCC-MLT patients undergoing the MS or IL procedure; the procedures were performed by one thoracic surgeon in our department between January 2007 and September 2013. The patients who underwent preoperative neo-adjuvant therapy or video-assisted thoracic surgery and whose esophagus was substituted by the jejunum or colon were excluded. Then, a total of 336 OSCC-MLT patients were categorized into the following two groups: an MS group with 188 patients and an IL group with 148 patients. The lymph node stations were numbered, and the T and N stages were assessed according to the definition by American Joint Committee on Cancer – Union for International Cancer Control (AJCC-UICC) edition 7. All patients were followed up until April 2015 or until death.
Surgical procedures
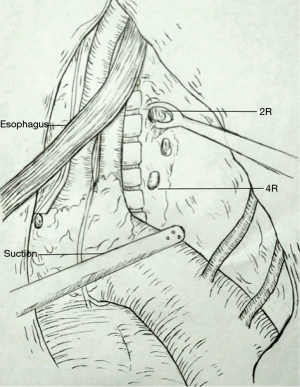
The IL procedure was carried out in a previously described standard method: above the aortic arch for esophagogastric anastomosis. The MS procedure was performed similar to the Sweet procedure, which occurs through the left posterolateral thoracotomy in the fifth or sixth intercostal, including esophagogastric anastomosis above the aortic arch. Systematic dissection was performed in upper abdominal and mid/lower mediastinal lymph nodes. However, for lymphadenectomy in the upper mediastina, the MS procedure as well as the IL procedure have respective advantages and disadvantages despite the superiority in the IL procedure. It is very difficult to systematically dissect the opposite lymph nodes in the upper mediastina. Therefore, a 2R or 4R dissection in the MS procedure and a 2L dissection via the right thorax were only completed by performing a sampling lymphadenectomy rather than a systematic lymph node dissection (Figure 1). Although resecting the 2L lymph nodes is slightly difficult because of the left subclavian obstruction and limited clearance above the aortic arch, resecting the 2R lymph nodes via the left thorax becomes feasible because its deeper position relatively enlarges the small clearance of the anatomy.
Propensity score matching
Propensity score matching was adopted to match subjects in the MS and IL groups to create a “quasi-random experiment” from retrospective data (14,15). Using a multivariable logistic regression model, we calculated the propensity scores by the covariates, including the gender, age, tumor location, G status, T status, and pathological stage. By the ‘Nearest Neighbor’ method, 1:1 matching algorithm, and caliper value 0.2, we matched and analyzed 129 MS patients (from 188 cases) and 129 IL patients (from 148 cases).
Evaluation of efficacy of lymph node dissection
The efficacy of lymph node dissection at each station was evaluated based on the index of estimated benefit from lymph node dissection (IEBLD), which is defined as the incidence of metastasis to each station (%) × 5-year OS rate of metastatic patients at the corresponding station (%)/100 (16). The incidence of metastasis to each station was calculated by the ratio between the number of patients with positive nodes at each station and the number of patients with dissected lymph nodes at the station. The cumulative 5-year OS rate of metastatic patients at each nodal station was calculated without any reference to other nodal stations. A higher index suggests a higher therapeutic value of lymph node dissection at this station.
Statistical analysis
The statistical analysis was performed on SPSS 22 (SPSS, Chicago, IL, USA). A two-sided P<0.05 was considered significant. The comparisons between two groups were analyzed with an independent samples t-test for continuous variables and with Pearson’s χ2 test and Fisher’s exact probability test for dichotomous variables. Continuous variables were expressed as the mean ± standard deviation. The survival curves were drawn by the Kaplan-Meier method, and survival rates between groups were compared using the log-rank test. The independent prognostic factors were identified by multivariate analyses of a stepwise Cox’s proportional hazard regression model.
Results
Patient characteristics
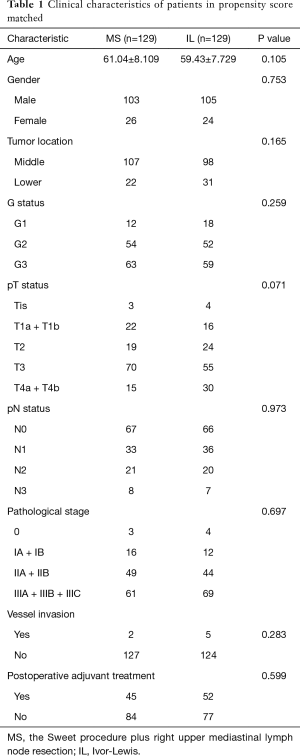
After 78 mismatching patients were excluded after matching from the total of 336 patients, 129 MS patients paired with 129 IL patients were involved in the final statistical analysis. The baseline characteristics were not significantly different between the groups (Table 1). All patients underwent curative surgery. These matching patients include 208 (80.6%) males and 50 (19.4%) females whose mean age was 60 years old (range, 37–80 years old). The mean number of lymphadenectomy per person was 20 (range, 1–62; total: 4,997) (MS: 22, IL, 17), and the mean number of lymph node metastases per person was 1.6 (range, 0–25; total: 405) (MS: 1.55, IL: 1.63). Approximately 124 of the 258 patients (48.1%) suffered from lymph node metastases.
Full table
Possible effect of lymph node dissection at each station
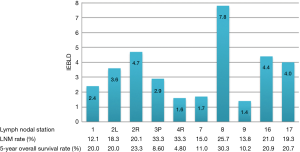
IEBLD was zero at stations 4L, 5, 6, 10, 10R, 15, 18, 19 and 20 because the 5-year OS rates were zero. Figure 2 shows the IEBLD and 5-year OS rates of metastatic patients and the incidence of metastasis at each station except for the stations with an IEBLD of zero. IEBLD was relatively high at stations 2L, 2R, 8, 16 and 17.
Incidence rates of lymph node dissection and metastasis
The comparison between the MS and IL groups for the incidence of lymph node dissection at each station is demonstrated in Table 2. Although the MS group performed worse in the dissection at stations 4R, 18, 20 (P=0.026, <0.001 and 0.004), it is more dominant at stations 2L, 4L, 5 and 6 (P<0.001, <0.001, – and –). There were no significant differences between the two groups at stations 2R, 8, 16 and 17 (P=0.794, 0.912, 0.452 and 0.836).
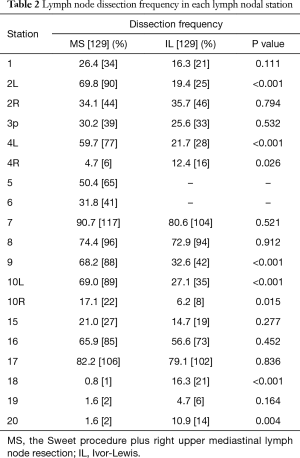
Full table
As shown in Table 3, neither the rates nor the ratios of lymph node metastasis in the other stations were different between the MS and IL groups, except for station 3p, which had a low total metastasis rate (8.6%) and ratio (6.1%), and stations 5 and 6, which only exist in the MS group. However, station 2R showed a high metastasis incidence at 25.0% in the MS group and 21.7% in the IL group.
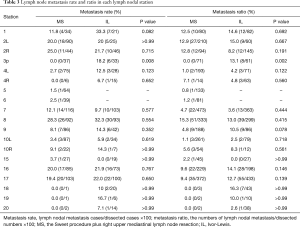
Full table
Postoperative outcomes
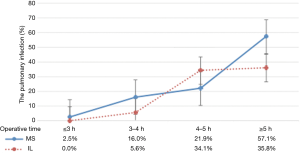
The overall incidence of complications was significantly lower in the MS group vs. the IL group (30.2% vs. 43.4%, P=0.028). The incidence rates of postoperative pulmonary complications were significantly higher in the IL group than the MS group (31.8% vs. 15.5%, P=0.002). As shown in Figure 3, the incidence of postoperative pulmonary complications increases with the longer operation time. However, the incidence rates of postoperative pulmonary complications during the same operative period were not significantly different between the MS and IL groups (Table 4).
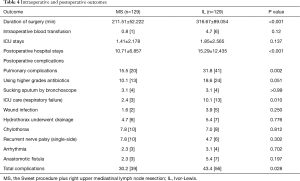
Full table
Survival analysis
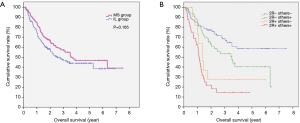
By the end of a median-term follow-up (median: 46.1 months, range from 19.3 to 99.2 months), 147 of the 336 patients survived, 165 patients died (49.1%) and 24 patients were missed (7.1%). The median survival time was 41.5 months, and the 5-year OS rate was 45.2% for the entire cohort. For the MS vs. IL patients, the corresponding values were 46.7 vs. 29.0 months and 48.1% vs. 42.5%, respectively; after matching, the corresponding values were 43.1 vs. 30.4 months and 46.9% vs. 44.0%, respectively, with no significant differences tested by univariate and multivariate analysis (P=0.165, 0.110) (Table 5 and Figure 4A). The age and G, T, and N stages are significantly associated with the survival of patients by multivariate Cox regression analysis with variables including the age, gender, tumor location, G, T, and N stages, vessel invasion, number of resected lymph nodes, surgical approach and postoperative adjuvant treatment.
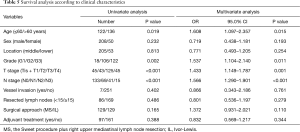
Full table
Discussion
The Sweet procedure, a conventional route for the resection of esophageal carcinoma, has received increasing criticism mainly focused on the lymphadenectomy, especially for RUM-LND. To address for this limitation, our department has performed the modified Sweet since early 2007. In this study, the number of lymph node dissections in each station was evaluated. After achieving quasi-random experimental matching by propensity score matching, we retrospectively identified the MS and IL procedures for OSCC-MLT. The MS versus IL procedure did not reduce the effective resection in each station of lymph nodes or the 5-year OS, but this procedure did shorten the operative time and postoperative in-hospital stay and decrease the incidence of postoperative complications in OSCC-MLT patients.
We used IEBLD to assess the therapeutic value of lymph node dissection at individual stations. With this index, Udagawa et al. conclude that the top five IEBLDs were 106recR, 106recL, 2 (lt. cardiac), 3 (lessor curvature) and 1 (rt. cardiac), as identified in 504 patients with middle thoracic esophageal carcinoma and in 1 (rt. cardiac), 3 (lessor curvature), 2 (lt. cardiac), 7 (lt. gastric artery) and 110 of 258 patients with lower thoracic esophageal carcinoma (17). Similarly, we identified lymph node stations 8, 2R, 16, 17 and 2L, which have high therapeutic values for patients (Figure 2). Although the names of the lymph node stations are different because of the two numbering systems (from Japan, AJCC), the represented regions are almost the same.
The actual value of RUM-LND for OSCC-MLT remains elusive and controversial (12,18). In our study, among all the resected lymph node stations, the IEBLD of 2R is only inferior to station 16 (Figure 2). Therefore, resecting 2R lymph nodes is beneficial for the treatment of OSCC-MLT. In addition, the metastasis rate of 2R was 25.0% in the MS group and 21.7% in the IL group. By extending the lymphadenectomy in the IL procedure, Stilidi et al. reported that the right paratracheal lymph node metastasis rates were 24.42% in middle thoracic esophageal carcinoma and 4.17% in lower thoracic (19), which are similar to our results. Station 2R represents the site of frequent lymph node metastasis and has a crucial role for obtaining more accurate N staging. Hsu et al. reported RUM-LNM as a poor prognosis in esophageal carcinoma patients (12). This finding is consistent with our results that the metastasis of 2R shows a poorer prognosis than other lymph node stations. The 5-year survival rates decrease successively in patients with N0, lymph node metastasis located only outside the right upper mediastina, lymph node metastasis located only in the right upper mediastina, and lymph node metastasis in both regions (Figure 4B). Neither the right upper mediastina lymph nodes, especially 2R, the lymph node metastasis rates, or the ratios are significantly different between two groups despite a smaller number of lymph node dissections in MS group than IL group. Those results are completely associated with the surgical method, namely, the MS patients who only underwent lymph node sampling resection rather than systematic lymphadenectomy in the right upper mediastina. Therefore, this study shows that 2R dissection for OSCC-MLT, similar to left thoracic sampling dissection, achieved an effective resection.
In the current study, the right thoracic approach is considered the gold standard for the resection of intrathoracic lymph nodes. Previous studies mostly report that the IL procedure is superior to the Sweet procedure with regard to lymph node dissection. However, by retrospectively identifying 748 Sweet patients and 167 IL patients, Ma et al. concluded that the Sweet and IL procedures are not significantly different in the number of the lymph node dissections or rate of metastasis (7). There are few reports comparing the IL and Sweet procedures among individual lymph node stations in detail. By comparing lymph nodes at each station between the two groups, we confirmed the results of Ma et al. The rates of the lymph node dissections, metastasis rates and ratios between the MS and IL procedures for individual lymph node stations were compared, which correspondingly represented the actual and effective dissections for those stations. The results revealed that the MS procedure is superior in stations 4L, 5 and 6 and inferior in stations 4R, 18 and 20 for lymph node dissection but that except for stations 3p, 5, 6, no differences were found in the lymph node metastasis rates or ratios in each regional station between the two procedures. These findings suggest that effective lymph node dissection of each station in MS is not inferior to the IL procedure.
Most previous studies show that the IL procedure presents a longer operating time, a longer in-hospital stay, and a higher incidence rates of operative complications than the Sweet procedure (6,9). These findings are consistent with our results showing that the IL procedure is featured with a significantly longer operative time and in-hospital stay. Furthermore, the rates of total postoperative complications are higher in the IL vs. the MS procedure (43.4% and 30.2%, respectively), especially postoperative pulmonary complications. Although the rates of other complications were not significantly different between the two procedures, the incidence rates of anastomotic fistula and incision infection were higher in the IL procedure. The incidence rates of postoperative pulmonary complications after open esophagectomy are reportedly more than 50% (20), which increases the mortality rate, prolongs the hospital stay and even causes poor long-term survival (21). As previously reported, a prolonged operative time is one of the strong risk factors for postoperative pulmonary complications because the rates of postoperative pulmonary complications increase gradually with a prolonged operative time (22). Despite the higher rates of postoperative pulmonary complications in the IL vs. the MS procedure (31.8% and 15.5%, respectively, P=0.02), no significant difference was found in the same operative time period, and the rate was even slightly lower in IL. Similar to previous studies, these findings suggest that prolonged single lung ventilation is associated with postoperative pulmonary complications (23). This finding could easily why the single lung ventilation process was performed through the entire operative procedure in MS patients. Therefore, single lung ventilation in the same operative time was shorter in IL patients than MS patients.
The existing relevant literature and meta-analyses of the Sweet vs. IL procedures suggest that the 5-year OS rates are not significantly different between the two procedures (6,7,9). In our series of OSCC-MLT patients, the 5-year OS rates between the MS and IL procedures were 46.9% and 44.0%, respectively, which are not prognostically different as assessed by univariate and multivariate analyses. The results demonstrate that the surgical approach is not a prognostic factor. Whether an extensive lymph node dissection could improve the 5-year OS rates is still under debate (10). Some studies indicate that extensive lymph node dissection could improve prognosis (24-26), but other studies state that it is not associated with better survival (11,27). In our study, patients with more than 15 resected lymph nodes did not show a survival advantage compared with patients with less than 15 resected lymph nodes. The possibility of improving survival with extensive lymph node dissection in OSCC-MLT patients remains unclear and needs further study.
This retrospective study has several limitations. First, the number of IL-treated patients was low. Despite enhancing comparability between the two groups after propensity score matching, the sample sizes in the two procedures decreased, especially in the MS group. Second, the relation with regard to the 2R dissection and 5-year OS in OSCC-MLT patients was not estimated because of the small sample size without resection for 2R, which needs confirmation by further research. In addition, we could not evaluate the status for recurrent laryngeal nerve lymph nodes because of the incongruences in naming the lymph node stations according to the definitions described by the AJCC manual.
In conclusion, the MS and IL procedures for OSCC-MLT are associated with similar lymphadenectomy and 5-year OS rates. However, the MS procedure outperforms the IL procedure with smaller incidence rates of postoperative complications, a shorter operative time and a shorter in-hospital stay. Therefore, the MS procedure of esophagectomy is not inferior to the IL procedure in efficiency but is safer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the Ethics Committee at the West China Hospital of Sichuan University (No. 201649).
References
- Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol 2016;41:88-95. [Crossref] [PubMed]
- Kato H, Nakajima M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg 2013;61:330-5. [Crossref] [PubMed]
- Dănilă N, Lupaşcu C, Andronic M, et al. Esophagectomy in esophageal cancer - is there an optimal approach? Chirurgia (Bucur) 2014;109:600-3. [PubMed]
- Neagoe RM, Sala D, Voidazan S, et al. Transthoracic versus Transhiatal esophagectomy: a permanent dilemma. our 15-year experience. Chirurgia (Bucur) 2013;108:780-7. [PubMed]
- Khullar OV, Jiang R, Force SD, et al. Transthoracic versus transhiatal resection for esophageal adenocarcinoma of the lower esophagus: A value-based comparison. J Surg Oncol 2015;112:517-23. [Crossref] [PubMed]
- Zhang H, Wang J, Wang W, et al. A meta-analysis of esophagectomy: the comparative study of Ivor-Lewis operation and Sweet operation. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:892-7. [PubMed]
- Ma J, Zhan C, Wang L, et al. The sweet approach is still worthwhile in modern esophagectomy. Ann Thorac Surg 2014;97:1728-33. [Crossref] [PubMed]
- Li B, Xiang J, Zhang Y, et al. Comparison of Ivor-Lewis vs Sweet esophagectomy for esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg 2015;150:292-8. [Crossref] [PubMed]
- Ma Q, Liu W, Long H, et al. Right versus left transthoracic approach for lymph node-negative esophageal squamous cell carcinoma. J Cardiothorac Surg 2015;10:123. [Crossref] [PubMed]
- Herbella FA, Laurino Neto RM, Allaix ME, et al. Extended lymphadenectomy in esophageal cancer is debatable. World J Surg 2013;37:1757-67. [Crossref] [PubMed]
- van der Schaaf M, Johar A, Wijnhoven B, et al. Extent of lymph node removal during esophageal cancer surgery and survival. J Natl Cancer Inst 2015.107. [PubMed]
- Hsu PK, Huang CS, Hsieh CC, et al. Role of right upper mediastinal lymph node metastasis in patients with esophageal squamous cell carcinoma after tri-incisional esophagectomies. Surgery 2014;156:1269-77. [Crossref] [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [Crossref] [PubMed]
- Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150-61. [Crossref] [PubMed]
- Inacio MC, Chen Y, Paxton EW, et al. Statistics in Brief: An Introduction to the Use of Propensity Scores. Clin Orthop Relat Res 2015;473:2722-6. [Crossref] [PubMed]
- Sasako M, McCulloch P, Kinoshita T, et al. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg 1995;82:346-51. [Crossref] [PubMed]
- Udagawa H, Ueno M, Shinohara H, et al. The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol 2012;106:742-7. [Crossref] [PubMed]
- Ye K, Xu JH, Sun YF, et al. Characteristics and clinical significance of lymph node metastases near the recurrent laryngeal nerve from thoracic esophageal carcinoma. Genet Mol Res 2014;13:6411-9. [Crossref] [PubMed]
- Stilidi I, Davydov M, Bokhyan V, et al. Subtotal esophagectomy with extended 2-field lymph node dissection for thoracic esophageal cancer. Eur J Cardiothorac Surg 2003;23:415-20. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Yamana I, Takeno S, Hashimoto T, et al. Randomized Controlled Study to Evaluate the Efficacy of a Preoperative Respiratory Rehabilitation Program to Prevent Postoperative Pulmonary Complications after Esophagectomy. Dig Surg 2015;32:331-7. [Crossref] [PubMed]
- Yang CK, Teng A, Lee DY, et al. Pulmonary complications after major abdominal surgery: National Surgical Quality Improvement Program analysis. J Surg Res 2015;198:441-9. [Crossref] [PubMed]
- Ma LY, Liao ZF, Wang GJ. Risk factors for post-surgical pulmonary complications in patients after esophagectomy for cancer: a multivariate logistic analysis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2011;23:625-8. [PubMed]
- Liu Q, Tan Z, Lin P, et al. Impact of the number of resected lymph nodes on postoperative survival of patients with node-negative oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg 2013;44:631-6. [Crossref] [PubMed]
- Morimoto J, Tanaka H, Ohira M, et al. The impact of the number of occult metastatic lymph nodes on postoperative relapse of resectable esophageal cancer. Dis Esophagus 2014;27:63-71. [Crossref] [PubMed]
- Zhu Z, Chen H, Yu W, et al. Number of negative lymph nodes is associated with survival in thoracic esophageal squamous cell carcinoma patients undergoing three-field lymphadenectomy. Ann Surg Oncol 2014;21:2857-63. [Crossref] [PubMed]
- Wong J, Weber J, Almhanna K, et al. Extent of lymphadenectomy does not predict survival in patients treated with primary esophagectomy. J Gastrointest Surg 2013;17:1562-8; discussion 1569. [Crossref] [PubMed]