Lazarus response to treatment of patients with lung cancer and oncogenic mutations in the intensive care unit
Background
Lung cancer is the leading cause of cancer-related mortality in recent years (1,2). Lung cancer is also the most common solid tumor in critically ill patients with cancer admitted to an intensive care unit (ICU) (3). Cooke et al. demonstrated a recent significant increase in admissions of patients with lung cancer to the ICU (4). The main reason for ICU admission is acute respiratory failure (3,5). Slatore et al. used data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare registry (1992 to 2007) and found that 65% of patients with lung cancer died within 6 months after ICU admission (5). Recently, Soares et al. evaluated data for 449 patients with lung cancer admitted in 22 ICUs in Europe and Latin America. Six-month survival rates were between 40% and 50% in patients with a non-progressive malignancy and good performance status (PS) (an Eastern Cooperative Oncology Group PS score ≤2). However, patients with progressive cancer and poor PS (PS score >2) had mortality rates >90% (6). Although outcomes of patients with lung cancer admitted to the ICU from different studies varied, the ICU mortality was around 50% (5-7).
The use of mechanical ventilation (MV) for patients who develop acute respiratory failure is associated with a mortality rate of over 70% (3,5,8). Treating patients with advanced non-small-cell lung cancer (NSCLC) using chemotherapy in the ICU is controversial for at least two reasons. First, a PS score >2 is considered to be a contraindication for chemotherapy administration. Second, NSCLC is usually less sensitive to chemotherapeutic drugs. Based on this, aggressive care for patients with progressive lung cancer is controversial (9).
However, not all types of advanced lung cancer display the same poor prognosis. In recent years, the treatment of NSCLC, especial adenocarcinoma, has undergone a paradigm change. The concept of targeted therapy has dramatically changed the management of NSCLC. Tumors that harbor epidermal growth factor receptor (EGFR) mutations can exhibit dramatic responses to an EGFR tyrosine-kinase inhibitor (TKI), such as gefitinib (10), erlotinib (11), or afatinib (12). Another example is tumors with c-ros oncogene 1 receptor tyrosine kinase (ROS1) mutations or chromosomal rearrangements of the anaplastic lymphoma kinase (ALK) gene that are very responsive to targeted therapy. The rapid and powerful effects of crizotinib (13,14), ceritinib (15), and alectinib (16) have also been identified.
Because the toxicity of molecular targeted therapy is less than that of cytotoxic agents, their use for patients with a poor PS and oncogenic mutated NSCLCs have been proven to be beneficial (17). In a landmark trial published in the Journal of Clinical Oncology, Inoue and colleagues showed that using gefitinib in 22 patients with NSCLC harboring EGFR mutations and PS scores ranging 3–4 showed an improvement in PS scores from 3–4 at baseline to 0–1 after treatment in nearly 70% of cases (17). The editor of the journal commented that molecular targeted therapy for patients with poor PS and NSCLC along with oncogenic mutations evokes a “Lazarus” response (18).
These findings increased the interest in targeted therapy for patients with NSCLC oncogenic mutations who were admitted to the ICU owing to a respiratory failure. In the past, the average ICU mortality rate was highest for patients with lung cancers compared with other solid cancers (19). Now, their prognosis may be changing. Molecular targeted therapy combined with best critical care practices may help improve PS, resulting in extubation and improved survival in this group of patients.
A few case reports and studies exist regarding the use of targeted therapy for patients with NSCLC in the ICU, such as the study recently published by Kerrigan et al. (8,20-27). Our perspective review describes in detail the most recently published data in order to highlight their findings and main pitfalls.
Literature review
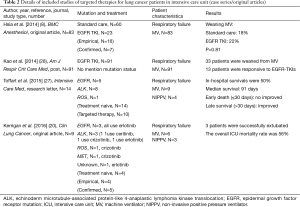
We scanned PubMed to identify studies reporting outcomes from targeted therapy in patients with oncogenic mutation advanced-stage NSCLC in the ICU via the following keywords “(lung cancer) AND (gefitinib) OR (erlotinib) OR (afatinib) OR (osimertinib) OR (crizotinib) OR (ceritinib) OR (alectinib) OR (EGFR) OR (ALK) OR (TKI) AND (intensive care unit) OR (ICU) OR (intensive care) OR (respiratory failure)”. We identified seven studies in addition to those published by Kerrigan et al. (8,20,22-27). We identified another journal article that was not included in PubMed (21) via an additional manual search (Tables 1 and 2). We also found two studies reporting outcomes for patients with respiratory failure (not necessarily in the ICU) (29,30).

Full table
Full table
In 2013, Ahn et al. first reported three critically ill patients with advanced adenocarcinoma and ALK translocations who required MV for respiratory failure and were successfully weaned from ventilators after treatment with ALK-inhibitors (21). In the last 4 years, there were 8 similar cases in ICU, including 2 tumors harboring EGFR mutations (22,23) and 6 harboring ALK mutations (21,24-26). Of these 8 patients, 2 were treated with empiric EGFR-TKI (erlotinib) in the ICU (22,23) and 1 patient was administered veno-venous extracorporeal membrane oxygenation (26) (Table 1). These reports suggest that ICU doctors require a new perspective on the use of molecular targeted therapy for patients with NSCLC and cancer related respiratory failure.
Based on these series of case reports, a French group led by Toffart assessed whether patients with advanced lung cancer harboring oncogenic mutations who are in critical condition should be admitted to ICUs (27) (Table 2). There were 14 patients with NSCLC who were oncogenic mutation treatment naive and were admitted to ICUs in 8 French hospitals between 2012 and 2014. The control group was selected by a method of matched samples from their previous study. Their mutations of 14 patients were observed in ROS1 (n=1), EGFR (n=5) and ALK (n=8). Of these patients, 10 (71%) received targeted therapy, 4 (29%) received noninvasive ventilation, and 9 (64%) patients received MV. Their in-hospital mortality rate was 50% (7/14). Median survival in the 14 patients was 91 days as compared with 10 days in the non-mutated group. Compared with the non-mutated group, the presence of oncogenic mutations had an insignificant impact on early death (≤30 days) but was associated with improved prolonged survival (hazard ratio 0.12; 95% confidence interval 0.03–0.47; P value =0.002).
More recently, Kerrigan and colleagues published an original study in Clinical Lung Cancer (20), which enrolled 9 patients with NSCLC receiving targeted TKI therapy who had been admitted to the ICU for acute respiratory failure requiring either invasive or noninvasive MV (Table 2). The mutation statuses of these 9 patients were as follows: 1 was unknown, 1 was MET, 1 was ROS1, 3 were EGFR, and 3 were ALK mutated. Four patients (4/9, 44%) were diagnosed on admission to the ICU and were treatment naive. Regarding their lung cancer treatment, 4 had received empiric EGFR-TKI for an unknown mutational status and of the remaining 5 patients, 2 continued with their TKI therapies, 2 started TKI therapy for the first time in the ICU, and the final patient received a second-generation ALK inhibitor due to previous treatment with a first-generation ALK inhibitor. Three patients (3/9, 33%) were successfully extubated; the second-generation ALK-inhibitor effectively stabilized disseminated intravascular coagulation in one case, and the patient was discharged to a rehabilitation facility. The remaining 5 patients showed no clinical response and were transferred to hospice care. The ICU mortality rate was 56%.
These authors concluded that patients with NSCLC in a critical condition and those with known or suspected oncogenic mutations should be considered for full-code life-sustaining treatment (20,27). Furthermore, in patients with NSCLC with an unknown mutation status and a high likelihood of harboring an EGFR mutation (never-smokers and Asian nonsquamous patients with NSCLC) (31), EGFR-TKI should be considered for empirical treatment, as a “shoot first, ask later” strategy (20,22,23). Physicians should also determine the mutation status if it is not known.
Appraisal of evidence
Novel targeted therapy modalities work in critically ill patients with NSCLC. However, questions remain despite these very encouraging results. Although a relatively large case series, the Kerrigan et al. study (20) has some limitations including that it was a retrospective medical record review in a single institution and has the possibility of many confounding variables that were not accounted for in the analysis of whether targeted therapy may benefit patients in the ICU. We know that a randomized trial to answer this question in terms of rescue therapy with molecular targeted therapy for oncogenic mutated patients with NSCLC requiring critical care will have ethical controversies. However, Kerrigan et al. (20) did not use a control group. Furthermore, although Toffart et al. used a matched group for further comparison (27), the study sample size (n=14) is relatively small for any meaningful statistical analysis. Another important point not emphasized in the study by Toffart and colleagues (27) was that of these 14 patients, 4 did not accept rescue targeted therapy. Furthermore, this study does not clarify which patient benefited from targeted therapy. In addition, the largest (n=83, 23 TKI users vs. 60 non-users) comparative effectiveness research by Hsia et al. reported “Rescue or maintenance therapy with EGFR TKI for stage IIIb–IV non-squamous patients with NSCLC requiring MV was not associated with better outcomes.” (8) (Table 2). Therefore, the beneficial effect of novel targeted therapies in critically ill patients with NSCLC is still unclear.
Determinants of short-term outcomes
We provided an interesting case here to illustrate the potential question of “lazarus response to treatment of patients with lung cancer and oncogenic mutations in the ICU”. A 42-year-old woman who had no history of smoking was admitted to the ICU because of bilateral disseminated lung cancer and pneumonia complicated with respiratory failure requiring MV support. Empirical antibiotics and adequate nutrition were administered. A bronchoscopy was performed along with a trans-bronchial biopsy. Empirical targeted therapy with EGFR-TKI was administered since day 3 of ICU admission. On the 10th hospital day, the patient was weaned off MV and was transferred to an ordinary ward. On the 14th hospital day, the transbronchial biopsy results indicated a lung adenocarcinoma with an ALK translocation. EGFR-TKI was discontinued and ALK inhibitors were prescribed. The patient had 10 months of progression-free survival when on the ALK inhibitor regimen and died 2 years later after multiple cycles of chemotherapy. Can we conclude EGFR-TKI is a powerful rescue drug for an ALK mutated lung adenocarcinoma patient in the ICU? The answer is probably “No” although we cannot definitely rule out this possibility (32).
Similar results were reported in a conference paper by Kao et al. (28) (Table 2). Of the enrolled 91 patients with NSCLC on MV taking EGFR-TKIs, 33 (36%) patients were successfully weaned from MV, which included 13 patients (13/33, 39%) who were responsive to EGFR-TKI treatment. In other words, 20 (20/33, 61%) patients without any response to targeted therapy could be successful weaned off MV.
In this scenario, one question is of utmost importance: to identify which patients will respond to treatment. Hsia’s group (8) designed a study for investigation of the issues and re-emphasized that the acute severity of patients with NSCLC with respiratory failure is the major factor associated with successful weaning from MV and mortality. They retrospectively collected data for 83 Asian patients in the ICU with advanced non-squamous NSCLC who required MV. Of these, 23 (23/83, 28%) patients took EGFR-TKIs and the others (60/83, 72%) accepted standard critical care. The demographic characteristics were balanced between the two groups of patients. Of the 83 patients, 16 (19%) were successfully weaned from the ventilator. The authors concluded that the use of EGFR-TKIs as rescue or maintenance therapy during respiratory failure did not improve the rate of successful weaning (standard care 18% vs. with EGFR TKIs, 22%; P value =0.81). Moreover, multiple logistic regression analyses showed that those with a lower acute severity score, via SAPS II (P value =0.03) or SOFA (P value =0.02), had higher rates of weaning from the ventilator (Table 2).
Undoubtedly, targeted therapy in patients with targetable oncogenic mutated NSCLC has a dramatic effect and leads to prolonged overall survival. However, these results may be significantly affected by organ dysfunction. When patients with NSCLC are admitted to the ICU owing to diverse critical conditions such as severe pneumonia, septic shock, or metastatic liver tumor induced hepatic failure, could add-on targeted drugs reverse the condition of multiple organ failure? Could targeted therapy still have the “Lazarus response” in patients with high acute severity scores?
Effect of targeted therapies on short-term & long-term outcomes
Has survival increased in patients with NSCLC cancer with oncogenic mutations who were admitted to the ICU after taking targeted therapy? By defending the pro viewpoint, Kerrigan et al. (20) and Toffart et al. (27) may be right in claiming that the survival had increased when targeted therapy was administered to the right patients. In the cons viewpoint, Hsia et al. (8) found that targeted therapy could not lead to a better rate of MV weaning or mortality. In Toffart’s analysis (27), targeted therapy also did not result in reduced early mortality (≤30 days) rates. The interesting finding by Toffart (27) is consistent with the results of Hsia’s study (8), that disease severity is the major determinant of outcomes in critically ill patients with NSCLC, not the cancer itself.
In the past, patients who have had a PS score of >2 at the time of discharge from the ICU had a worse long-term survival because these patients were unable to accept scheduled chemotherapy or radiotherapy (33). However, in the era of targeted therapy, this problem is no longer an issue in patients with oncogenic mutated NSCLC. Therefore, patients with NSCLC with oncogenic mutations could benefit from targeted therapies, but only if they survive the critical period in the ICU because of the acute illness wherein the severity determined by many factors in addition to the lung cancer. Because targeted therapy could partial resolve the problems of underlying lung cancer, the choice to accept full intensive medicine should take into account the wishes of the patient, trajectory of the patient’s multiple organ condition, and the expected long-term quality of life. We cannot over-emphasize or be over-optimistic about the dramatic effect of targeted therapy regarding short-term outcomes and most physicians have not realized that multiple organ function has been confirmed as a major determinant of patient prognosis.
Conclusions
Undoubtedly, there is a population of oncogenic mutated patients with NSCLC requiring ICU support who can benefit from targeted therapy. Although the long-term prognosis is determined by characteristics of the underlying NSCLC, the short-term outcome is mainly determined by the severity of the acute illness.
Acknowledgements
None.
Footnote
Provenance: This is an invited Perspective commissioned by the Section Editor Xue-Zhong Xing [National Cancer Center (NCC)/Cancer Hospital, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC), Beijing, China].
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Adam AK, Soubani AO. Outcome and prognostic factors of lung cancer patients admitted to the medical intensive care unit. Eur Respir J 2008;31:47-53. [Crossref] [PubMed]
- Cooke CR, Feemster LC, Wiener RS, et al. Aggressiveness of intensive care use among patients with lung cancer in the Surveillance, Epidemiology, and End Results-Medicare registry. Chest 2014;146:916-23. [Crossref] [PubMed]
- Slatore CG, Cecere LM, LeTourneau JL, et al. Intensive care unit outcomes among patients with lung cancer in the surveillance, epidemiology, and end results–Medicare registry. J Clin Oncol 2012;30:1686-91. [Crossref] [PubMed]
- Soares M, Toffart AC, Timsit JF, et al. Intensive care in patients with lung cancer: a multinational study. Ann Oncol 2014;25:1829-35. [Crossref] [PubMed]
- Roques S, Parrot A, Lavole A, et al. Six-month prognosis of patients with lung cancer admitted to the intensive care unit. Intensive Care Med 2009;35:2044-50. [Crossref] [PubMed]
- Hsia TC, Tu CY, Chen HJ. The impact of rescue or maintenance therapy with EGFR TKIs for Stage IIIb-IV non-squamous non-small-cell lung cancer patients requiring mechanical ventilation. BMC Anesthesiol 2014;14:55. [Crossref] [PubMed]
- Benoit DD, Soares M, Azoulay E. Has survival increased in cancer patients admitted to the ICU? We are not sure. Intensive Care Med 2014;40:1576-9. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or Carboplatin-Paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Inoue A, Kobayashi K, Usui K, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 2009;27:1394-400. [Crossref] [PubMed]
- Langer CJ. The "Lazarus Response” in treatment-naïve, poor performance status patients with non-small-cell lung cancer and epidermal growth factor receptor mutation. J Clin Oncol 2009;27:1350-4. [Crossref] [PubMed]
- Puxty K, McLoone P, Quasim T, et al. Survival in solid cancer patients following intensive care unit admission. Intensive Care Med 2014;40:1409-28. [Crossref] [PubMed]
- Kerrigan K, Shoben A, Otterson G.. Treatment of Lung Cancer Patients With Actionable Mutations in the Intensive Care Unit. Clin Lung Cancer 2016;17:523-7. [Crossref] [PubMed]
- Ahn HK, Jeon K, Yoo H, et al. Successful Treatment with Crizotinib in Mechanically Ventilated Patients with ALK Positive Non-Small-Cell Lung Cancer. J Thorac Oncol 2013;8:250-3. [Crossref] [PubMed]
- Jeong SH, Um SW, Lee H, et al. Successful Treatment with Empirical Erlotinib in a Patient with Respiratory Failure Caused by Extensive Lung Adenocarcinoma. Korean J Crit Care Med 2016;31:44-8. [Crossref]
- Bosch-Barrera J, Sais E, Lorencio C, et al. Successful empirical erlotinib treatment of a mechanically ventilated patient newly diagnosed with metastatic lung adenocarcinoma. Lung Cancer 2014;86:102-4. [Crossref] [PubMed]
- van Geffen WH, Hiltermann TJ, Groen HJ. Surviving respiratory insufficiency with intensive care support in a pretreated, extensively metastasized patient with an EML4-ALK translocation. J Thorac Oncol 2013;8:e1-e2. [Crossref] [PubMed]
- Tanaka H, Taima K, Morimoto T, et al. Dramatic response to alectinib in a patient of ALK-rearranged lung cancer with poor performance status. BMC Res Notes 2016;9:173. [Crossref] [PubMed]
- Adam V, Dooms C, Vansteenkiste J. Lung cancer at the intensive care unit: The era of targeted therapy. Lung Cancer 2015;89:218-21. [Crossref] [PubMed]
- Toffart AC, Dhalluin X, Girard N, et al. Patients with advanced lung cancer harboring oncogenic mutations should be admitted to intensive care units. Intensive Care Med 2015;41:164. [Crossref] [PubMed]
- Kao K, Tsai Y, Hu H, et al. The Predictors Of Successful Weaning From Mechanical Ventilation Of Non-Small Cell Lung Cancer Patients Receiving Tyrosine Kinase Inhibitors In Intensive Care Units. Am J Respir Crit Care Med 2014;189:A6697.
- Lee CH, Liam CK, Pang YK, et al. Successful pregnancy with epidermal growth factor receptor tyrosine kinase inhibitor treatment of metastatic lung adenocarcinoma presenting with respiratory failure. Lung Cancer 2011;74:349-51. [Crossref] [PubMed]
- Yoshida T, Hida T, Yatabe Y. Rapid and dramatic response to alectinib in an anaplastic lymphoma kinase rearranged non-small-cell lung cancer patient who is critically ill. Anticancer Drugs 2016;27:573-5. [Crossref] [PubMed]
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37. [Crossref] [PubMed]
- Alì G, Chella A, Lupi C, et al. Response to erlotinib in a patient with lung adenocarcinoma harbouring the EML4-ALK translocation: a case report. Oncol Lett 2015;9:1537-40. [PubMed]
- Caruso P, Ferreira A, Laurienzo C, et al. Short-and-long-term survival of patients with metastatic solid cancer admitted to the intensive care unit: prognostic factors. Eur J Cancer Care (Engl) 2010;19:260-6. [Crossref] [PubMed]


