The role of transforming growth factor (TGF)-β in the infarcted myocardium
Introduction
Transforming growth factor (TGF)-β is the prototypical member of a large family of secreted growth factors; in mammals, the TGF-β family is comprised of 33 members (1). Originally identified as a cytokine that induces cellular “transformation” in vitro, promoting anchorage-independent growth (2-5), TGF-β is now recognized as a pleiotropic and multifunctional growth factor that regulates a wide range of cellular responses, and may play a critical role in development, and in the pathogenesis of many diseases (1,6,7). In mammals, three TGF-β isoforms (β1, β2 and β3) have been identified; these isoforms are encoded by different genes. Although in vitro studies suggest that the three isoforms have similar actions, the distinct phenotypes of mice with isoform-specific genetic disruption indicate numerous non-compensated functions of the three TGF-βs in organ development and in tissue homeostasis (8-11). Despite a large body of evidence demonstrating induction, release and activation of TGF-βs following tissue injury, understanding of their in vivo functions in repair and remodeling has been hampered by the complexity of their context-dependent actions and downstream signaling cascades.
The adult mammalian heart has negligible regenerative capacity. Following myocardial infarction up to one billion cardiomyocytes become necrotic; this massive sudden loss of cardiomyocytes overwhelms any existing regenerative reserve, resulting in replacement of dead cells with collagen-based scar. The cellular events involved in repair of the infarcted heart can be divided in three distinct, but overlapping phases: the inflammatory phase, the proliferative phase and the maturation phase (12,13). During the inflammatory phase, release of alarmins by necrotic cardiomyocytes activates innate immune pathways, leading to recruitment of leukocytes in the infarcted myocardium (14). Clearance of the infarct from dead cells and matrix debris by professional phagocytes activates anti-inflammatory cascades leading to suppression of the inflammatory response and transition to the proliferative phase of infarct healing (15). During the proliferative phase, activated myofibroblasts deposit large amounts of extracellular matrix proteins in the infarcted area, while activation of angiogenesis ensures perfusion of the highly cellular and metabolically active wound. The maturation phase follows, as activation of anti-fibrotic pathways limits the fibrogenic response, leading to formation of a mature scar with low cellular content that contains cross-linked collagenous matrix. Healing of the infarcted heart is associated with adverse remodeling of the ventricle, a constellation of cellular events that involve both infarcted and non-infarcted segments and are associated with the development of heart failure (16).
A growing body of evidence suggests that TGF-β signaling pathways play an important role in regulation of the cellular events associated with cardiac repair, by modulating injurious, inflammatory, reparative, angiogenic, and fibrogenic responses. This manuscript reviews our current understanding of the role of TGF-β in the infarcted heart, focusing on its diverse cellular actions, discussing molecular cascades modulated by TGF-β, and identifying potential therapeutic targets.
Expression of TGF-β in normal myocardium
High levels of myocardial TGF-β expression have been observed during embryonic development and in adult life, predominantly localized in cardiomyocytes and in the extracellular matrix (17). TGF-βs have been implicated in cardiac development and in valve morphogenesis (18,19). In adult mammals, TGF-β is stored in the myocardium in a latent form; whether low-level constitutive TGF-β activity is important for cardiac function remains unknown. In neonatal rat cardiac cardiomyocytes, exogenous TGF-β sustained spontaneous rhythmic beating in serum-free conditions (20); however, the in vivo relevance of these observations is unclear.
Regulation of TGF-β isoforms in myocardial infarction
Induction of TGF-β isoforms has been extensively documented in both mouse and large animal models of myocardial infarction (17,21-26). In reperfused mouse infarcts, TGF-β1 and β2 mRNA levels show an early peak after 6–72 h of reperfusion; in contrast, TGF-β3 upregulation exhibits a prolonged time course with persistently elevated expression after 7 days of reperfusion (21). Most cell types involved in cardiac repair are capable of synthesizing and secreting large amounts of TGF-β; the relative contributions of various cell types in infarction-related upregulation remains poorly defined. Whether different cell types and distinct molecular pathways are responsible for upregulation of each TGF-β isoform is unknown. Studies in a porcine model of chronic coronary constriction suggested that cardiomyocytes are a major source of TGF-β following cardiac injury (22). On the other hand, experiments in a mouse model of myocardial infarction suggested that TGF-β is localized in infarct macrophages (27). Genetic disruption of monocyte chemoattractant protein (MCP)-1/CCL2, a chemokine with a crucial role in recruitment of monocytes/macrophages in the infarcted myocardium attenuated TGF-β2 and β3 mRNA expression, suggesting that mononuclear cells may be an important source of TGF-β isoforms in reperfused infarcts (28). The contributions of other cell types have not been convincingly documented. Platelets have been suggested to be an important source of TGF-β1 in the pressure-overloaded myocardium (29); however, their role as a source of growth factors in the infarcted myocardium has not been systematically investigated. Lymphocyte subsets and mast cells infiltrate the infarcted heart (30-33) and are capable of producing TGF-βs. Moreover, much like most tissues, the normal myocardium may contain constitutive stores of matrix-bound latent TGF-β that can be activated following injury (34), initiating a response even in the absence of de novo synthesis.
The biology of TGF-β activation
TGF-βs are secreted as latent complexes, consisting of the TGF-β dimer, the latency-associated peptide (LAP), and a latent TGF-β-binding protein (LTBP). Current concepts suggest that while the LAP confers latency to TGF-β (35,36), the LTBP contributes to sequestration into the extracellular matrix (34). Although the LAP is cleaved intracellularly from the mature TGF-β dimer through furin-mediated actions, TGF-β and LAP remain bound after secretion through non-covalent interactions, forming the small latent complex. Activation of TGF-β signaling cascades requires release of the TGF-β dimer from the latent complex and requires the co-operation of several distinct mediators, including integrins, proteases, reactive oxygen species (ROS) and matricellular proteins.
In the infarcted heart, evidence suggesting the presence of bioactive TGF-β in cardiac extracellular fluids (37), and activation of downstream Smad-dependent signaling (38) suggest rapid activation of TGF-β. The mechanisms responsible for TGF-β activation following infarction remain poorly understood. ROS activation is a hallmark of the ischemic response and may be involved in TGF-β activation in the infarcted myocardium. Cell surface integrins interact with LAP and have been directly implicated in TGF-β activation in many tissues (39); however, whether integrins play an important role in activating TGF-β in the infarcted heart remains unknown. Although in vitro, αvβ5 and αvβ3 integrins contributed to latent TGF-β activation and subsequent cardiac myofibroblast differentiation (40); the in vivo significance of these interactions has not been directly tested. Proteases of various classes (including serine proteases, cathepsins, metalloproteinases, and cysteine proteases) are capable of activating TGF-β in vitro. Although protease release and activation during the inflammatory phase of infarct healing may contribute to TGF-β activation; the in vivo significance of these effects has not been investigated. Matricellular proteins are markedly upregulated in the infarcted heart (41-43) and may play an important role in TGF-β activation. The prototypical matricellular protein thrombospondin (TSP)-1 interacts with LAP, promoting release of the TGF-β dimer from the latent complex (44). In both mouse and canine infarcts, a strikingly selective upregulation of TSP-1 in the infarct border zone is associated with activation of TGF-β signaling. TSP-1 loss attenuates activation of Smad-dependent pathways following myocardial infarction, suggesting an important role for this matricellular protein in activation of TGF-β cascades (41). Finally, exposure to an acidic environment has been implicated in activation of latent TGF-β. It has been suggested that lactic acid may trigger pH-dependent TGF-β activation in patients with idiopathic pulmonary fibrosis (45). Whether the abundant lactic acid generated in the ischemic myocardium is involved in local activation of TGF-β remains unknown.
Cellular actions of TGF-β in the infarcted myocardium
TGF-βs are capable of regulating phenotype and function of all cell types involved in cardiac injury and repair (Figure 1). Although TGF-β1, β2 and β3 exhibit distinct patterns of regulation following myocardial infarction, information on isoform-specific actions in the infarcted myocardium is lacking. Most in vitro studies have investigated TGF-β1-mediated actions. In vivo experiments on the other hand, have focused on the role of TGF-β receptor-activated signaling, exploring pathways common to all three isoforms and to other members of the TGF-β superfamily. Thus, our current knowledge precludes conclusions regarding cellular actions of specific isoforms.
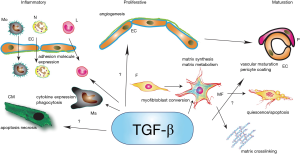
Effects of TGF-β on ischemic cardiomyocytes
Early studies suggested that injection of exogenous TGF-β in isolated perfused hearts undergoing brief myocardial ischemia followed by reperfusion exerts protective actions, attenuating oxidative stress and reducing release of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α (46). In a model of reperfused feline myocardial infarction, TGF-β1 administration reduced cardiomyocyte death; these protective actions were associated with attenuated neutrophil recruitment in the infarcted myocardium (47). Whether TGF-β-mediated protection in these studies is due to activation of direct pro-survival pathways in cardiomyocytes, or reflects suppression of injurious inflammatory signaling, remains unknown. Regarding the effects of TGF-β on cardiomyocyte survival, ex vivo and in vitro experiments have produced conflicting results. In isolated perfused hearts, TGF-β1 infusion during early reperfusion protected cardiomyocytes from apoptosis through actions involving p42/p44 mitogen-activated protein kinase (MAPK) signaling (48). In contrast, in rat cardiomyocytes the pro-apoptotic effects of angiotensin II were attributed to activation of TGF-β signaling (49). Moreover, in vitro studies have demonstrated direct actions of TGF-β on cardiomyocyte function, mediated through upregulation of the laminin receptor 37/67 (50). Considering its notoriously pleiotropic and context-dependent actions, the effects of exogenous administration of TGF-β are likely dependent on the dose, route and timing of administration, and the specific characteristics of the experimental model.
Endogenous cardiomyocyte-specific TGF-β actions have been recently investigated using genetic models of cell-specific TGF-β receptor disruption (51). In a model of non-reperfused myocardial infarction, mice with cardiomyocyte-specific disruption of TGF-β signaling were protected from death due to cardiac rupture. The mechanism of protection remains poorly defined. It was suggested that TGF-β signaling in cardiomyocytes may suppress synthesis of cardioprotective genes, such as interleukin (IL)-33, growth differentiation factor (GDF)-15 and TSP-4.
Effects of TGF-β on immune cells
A large body of in vitro and in vivo evidence suggests that TGF-βs modulate phenotype and function of immune cells, critically regulating inflammatory responses (52). In vitro, femtomolar concentrations of TGF-β promote neutrophil (53) and monocyte (54) chemotaxis; this effect may be important for recruitment of leukocytes in inflamed tissues. Picomolar TGF-β concentrations stimulate synthesis of a variety of pro-inflammatory cytokines and chemokines by monocytes (54,55). In contrast to its pro-inflammatory actions in monocytes, TGF-β is known to deactivate macrophages, suppressing MCP-1/CCL2, IL-1β and TNF-α synthesis (56-58). It should be emphasized that effects of TGF-β on immune cells can be either pro- or anti-inflammatory depending on the cytokine milieu, the tissue origin of the cells, and the experimental context (59). For example, despite its potent chemotactic actions for monocytes and neutrophils in single-cell assays, TGF-β may attenuate leukocyte migration across an endothelial layer by reducing surface expression of adhesion molecules (60). Thus, attribution of pro- or anti-inflammatory properties to TGF-βs should be based on robust in vivo evidence, rather than on in vitro experiments.
In vivo, TGF-β1 plays an essential role in preventing spontaneous inflammation in mammalian tissues. Approximately 50% of TGF-β1 null mice develop normally and show no gross developmental abnormalities. However, 2–4 weeks after birth, these mice develop massive multi-organ inflammation, predominantly affecting the heart and lungs (9). Mice with T cell-specific loss of the type II TGF-β receptor TβRII exhibited an inflammatory disease with striking similarities to TGF-β1 KO animals, suggesting that TGF-β may act by suppressing T cell-mediated inflammation (61).
Repair of the infarcted heart is dependent on timely recruitment of inflammatory leukocytes, and subsequent activation of reparative macrophages that stimulate myofibroblast activation and angiogenesis (15,62). Evidence from in vitro studies and in vivo neutralization experiments suggest that, in healing infarcts, TGF-β may regulate inflammatory leukocyte function. Experiments in a canine model of myocardial infarction demonstrated that TGF-β bioactivity is markedly increased in the post-ischemic cardiac lymph (reflecting release of active TGF-β in the cardiac extracellular space), and contributes to monocyte chemoattractant activity during the first five hours of reperfusion (37). In a mouse model of non-reperfused myocardial infarction, early systemic inhibition of TGF-β signaling through transfection with the extracellular domain of TβRII increased mortality, accentuating neutrophil recruitment, and increasing expression of TNF-α, IL-1, and MCP-1/CCL2 (63). These observations are consistent with an important role for TGF-β in negative regulation of the post-infarction inflammatory response. In addition, mice with genetic loss of TSP-1, a crucial TGF-β activator, had defective containment of the post-infarction inflammatory response, associated with evidence of attenuated Smad2 activation (41). These effects may reflect a crucial role for localized TSP-1-mediated activation of TGF-β in the infarct border zone as a “barrier” preventing expansion of the inflammatory reaction into the non-infarcted area. In the absence of TSP-1, expansion of inflammatory activation increases fibrosis and accentuates adverse remodeling (41). However, it should be emphasized that TSP-1 has multiple cellular targets and several functional domains and may regulate inflammation and fibrosis through TGF-β-independent effects (64).
The specific effects of TGF-β on the phenotype of immune cells in the infarcted myocardium remain poorly understood. In vitro, TGF-β has been shown to modulate macrophage phenotype, promoting M2 polarization (65), enhancing macrophage-colony stimulating factor (M-CSF)-induced proliferation (59), inhibiting nitrite release (66), reducing cytotoxic activity (67), and suppressing release of inflammatory mediators (68). However, the potential involvement of TGF-β in mediating the dynamic phenotypic alterations of infarct macrophage subpopulations remains unknown. TGF-β also serves as a central mediator in T lymphocyte differentiation and activation, critically regulating phenotype and function of all subpopulations (69-71). Although activated subsets of T lymphocytes have been implicated in repair and remodeling of the infarcted heart (30,32,72), the relative role of TGF-β signaling in their recruitment and activation has not been investigated.
TGF-β in regulation of fibroblast phenotype and function
The central role of TGF-β in cardiac fibroblast activation is well-documented by a wide range of in vitro studies and by extensive associative in vivo evidence (73). TGF-β mediates conversion of fibroblasts into myofibroblasts (74), a phenotypic transformation associated with reparative and fibrotic responses. Moreover, TGF-β markedly and consistently stimulates synthesis of extracellular matrix proteins (such as collagen I, collagen III and fibronectin) (75,76) and promotes a matrix-preserving program by decreasing collagenase expression and by accentuating tissue inhibitor of metalloproteinases (TIMP)1 expression (38,77). In contrast, effects of TGF-β on cardiac fibroblast proliferation are less consistent: both proliferative and anti-proliferative effects have been reported (76,78). Differences in TGF-β concentration, and in the experimental context may account for the conflicting observations.
During the proliferative phase of infarct healing, abundant myofibroblasts infiltrate the infarct border zone (79,80) and serve as the main source of collagen (81), while participating in extracellular matrix metabolism by secreting matrix metalloproteinases (MMPs). Most infarct myofibroblasts originate from resident fibroblast populations (82,83); TGF-β may play a crucial role in conversion of these highly plastic interstitial cells into myofibroblasts. TGF-β may also stimulate extracellular matrix synthesis and modulate the protease expression profile of these cells. Unfortunately, this concept is only supported by systemic TGF-β inhibition experiments; in vivo effects of fibroblast-specific loss of TGF-β signaling have not been studied. In a model of non-reperfused infarction, TGF-β inhibition through administration of a neutralizing antibody had detrimental effects, accentuating chamber dilation, increasing myocardial MMP expression, and reducing collagen synthesis (84). Two independent investigations inhibiting TGF-β by using gene transfer of the extracellular domain of TβRII suggested that TGF-β may play a key role in fibrosis of the infarcted heart (63,85). TGF-β inhibition after the inflammatory phase of cardiac repair attenuated deposition of fibrous tissue in the infarcted region (85). These investigations support the critical role of TGF-β as a regulator of extracellular matrix deposition and metabolism following myocardial infarction. However, whether fibroblasts are the main cellular targets responsible for these actions of TGF-β remains unknown.
Effects of TGF-β on endothelial cell phenotype and on infarct angiogenesis
Vascular endothelial cells are the most abundant non-cardiomyocytes in the adult mammalian myocardium (86), and play an important role in repair of the infarcted heart. During the inflammatory phase, endothelial cells serve as a source of chemokines (87,88); interactions between activated endothelial cells and leukocytes are critical for recruitment of neutrophils and monocytes in the infarcted myocardium (89). During the proliferative phase, endothelial cells proliferate and generate neovessels (90), important for perfusion of the healing infarct and for supply of granulation tissue cells with oxygen and nutrients. Finally, during the maturation phase, neovessels acquire a coat comprised of mural cells (91,92); this process restrains angiogenesis, suppresses inflammatory activation, and may contribute to stabilization of the scar (93).
TGF-β is critically involved in vascular development through effects on both endothelial cells and pericytes (94) and modulates endothelial cell gene expression and activity. The actions of TGF-β on endothelial cells may be either angiogenic or angiostatic, depending on the context, the differentiation of the cells, and the presence or absence of other mediators (94,95). Very limited information is available on the effects of TGF-β on vascular cells in the infarcted myocardium. In vitro, TGF-β stimulation attenuates chemokine synthesis by cytokine-stimulated endothelial cells (96); such actions may contribute to suppression of the inflammatory response following myocardial infarction. TGF-β may also be implicated in infarct angiogenesis and vascular maturation; however, information on specific cellular actions is lacking.
TGF-β and cardiac regeneration
Enhancement of the extremely limited regenerative capacity of the infarcted heart is a major goal in cardiovascular research (97,98). Because of their broad effects on differentiation and fate of progenitor cells (99), several members of the TGF-β family have been suggested as potential activators of the regenerative response. Fish and amphibians exhibit robust myocardial regenerative responses; in zebrafish, TGF-β has been implicated in myocardial regeneration (100). In vitro, TGF-β stimulation increased the expression of cardiac transcription factors in embryonic stem cells, directing them towards cardiomyocyte differentiation (101). In vivo, implantation of TGF-β pre-programmed CD117+ stem cells into the infarcted myocardium induced angiogenesis and was reported to promote a regenerative response (102). However, in other studies, TGF-β appeared to act as a suppressor of cardiac regeneration. TGF-β inhibition enhanced differentiation of stem cell-derived mesoderm to cardiomyocytes (103). Moreover, in a model of cardiac injury, TGF-β inhibition improved cardiomyoblast-mediated regeneration (104). The conflicting findings may reflect distinct effects of TGF-β on various cell types used to promote myocardial remuscularization.
TGF-β signaling pathways in the infarcted myocardium
Active TGF-β signals by binding to the TGF-β receptor complex at the cell surface. TGF-β binds to the constitutively active TβRII; this complex recruits and transphosphorylates the type I receptor (TβRI) (105,106). TβRI activation propagates downstream signaling involving a family of intracellular effectors, the Smad proteins (106,107). The receptor activated Smad proteins (R-Smads), Smad2 and Smad3 are phosphorylated upon activation of the cytoplasmic domain of TβRI; then form a trimeric complex with the common Smad, Smad4. Subsequently, the activated Smad complex translocates to the nucleus, recruits coactivators or corepressors into transcriptional complexes (108), and regulates gene transcription. Although TGF-βs typically activate Smad2/3 cascades, it has been recognized that Smad1 and Smad5 may also be activated by TGF-β in certain cell types, providing an alternative Smad-dependent pathway for signal transduction (109). The structurally divergent inhibitory Smads (i-Smads), Smad6 and Smad7, are induced by TGF-βs as part of a negative feedback loop that inhibits TGF-β signaling by interfering with phosphorylation of R-Smads (110). In addition to Smad-dependent signaling, TGF-β also activates a wide range of non-canonical cascades, including p38 MAPK, Erk, JNK, TAK-1, and Rho GTPase pathways (111-113).
Activation of both Smad2/3 and Smad1/5 pathways has been reported in infarcted hearts (38,114). Although TGF-βs are likely important activators of the Smad2/3 cascade, other members of the TGF-β family, angiotensin II, or matricellular proteins may also contribute to Smad activation. Our knowledge on the role of Smad3 signaling in myocardial infarction is based on in vitro experiments and on investigations using mice with global loss of Smad3. Smad3 KO animals had no significant defects in resolution of inflammation; but exhibited attenuated leukocyte recruitment in the infarcted area. Moreover, global Smad3 loss reduced fibrosis in the infarct border zone and in the remodeling myocardium, resulting in improved diastolic function (38). In vitro experiments showed that Smad3 null cardiac fibroblasts were hyperproliferative, but exhibited impaired function. Smad3 was critically involved in myofibroblast transdifferentiation and mediated TGF-β-induced extracellular matrix synthesis and TIMP upregulation (38,76). Considering the broad effects of Smad3 on all cell types, it is unclear whether the improved remodeling exhibited by mice with global loss of Smad3 is due to fibroblast-mediated actions. Understanding the role of Smad-dependent signaling in myocardial infarction requires cell-specific loss-of function strategies.
The role of Smad-independent TGF-β signaling pathways in the infarcted heart has not been systematically investigated. TGF-β-activated kinase (TAK)-1, a member of the MAPK family, is activated in the pressure-overloaded myocardium, and is involved in cardiac hypertrophy and fibrosis (115). The potential role of TAK-1 following infarction has not been studied. Experimental studies in models of renal and pulmonary injury have suggested an important role for non-Smad pathways [such as p-21-activated kinase 2 (PAK2) and c-Abl] in fibrotic diseases (116,117). However, the involvement of these pathways in repair and fibrosis of the infarcted heart remains unknown.
It has been proposed that, in addition to its direct effects on the cells responsible for cardiac repair, TGF-β may also act by inducing expression of downstream effectors, such as the matricellular protein connective tissue growth factor (CTGF)/CCN2, or endothelin (118). CCN2 may synergize with TGF-β to stimulate cardiomyocyte hypertrophy (119) and enhance fibrosis (76). Although transgenic overexpression studies have suggested protective effects of CCN2 on the size of the infarct (120), actions of endogenous CCN2 in the infarcted myocardium have not been dissected. It is unclear, whether any of the effects of TGF-β on the cellular response to myocardial infarction are mediated through CCN2. A recent study suggested that in a model of myocardial TGF-β overactivation, induced through cardiac-specific expression of an active TGF-β mutant, CCN2 did not play an important role in cardiac pathology (121).
Targeting TGF-β in myocardial infarction
Because of its critical role in repair, remodeling, fibrosis and regeneration, TGF-β is considered an attractive therapeutic target in myocardial infarction and cardiac remodeling (118). Unfortunately, the pleiotropic and context-dependent actions of the TGF-β isoforms, and the complexity of TGF-β-activated signaling cascades have hampered therapeutic application. Clearly, in the infarcted myocardium, TGF-β has both beneficial and detrimental actions. Identification of safe and effective therapeutic strategies will require understanding of the cellular basis for these effects, and dissection of the distinct actions of Smad-dependent and Smad-independent TGF-β signaling. Unfortunately, expansion of our knowledge on the pathophysiological role of TGF-β in myocardial infarction may not be sufficient for implementation of effective strategies in human patients. In the clinic, post-infarction remodeling and heart failure is pathophysiologically heterogeneous. For the same amount of cardiomyocyte loss, some patients develop dilative remodeling and systolic dysfunction, while others may have extensive fibrosis, accompanied by diastolic heart failure (122). Differences in TGF-β responses between patients may account for the distinct patterns of post-infarction remodeling in various subpopulations. Identification of patients with overactive TGF-β responses through the use of carefully validated biomarkers, or imaging studies, will be important in order to design personalized treatment strategies. Moreover, it should be emphasized that, because of its broad effects on many cell types, targeting TGF-β may carry significant risks (123). A large body of evidence suggests that disruption of the TGF-β/Smad axis promotes aortic aneurysm formation and rupture (124-126). Thus attempts for clinical translation of TGF-β/Smad inhibition strategies should exclude patients vulnerable to the potentially adverse consequences of Smad3 disruption on vascular remodeling. Temporal considerations are also highly significant: early inhibition of the TGF-β response may perturb reparative responses essential to maintain the structural integrity of the ventricle. Successful translation will require a combination of animal studies to understand the cellular targets and functions of TGF-β, and human investigations to identify patients likely to benefit from specific therapeutic interventions.
Acknowledgements
Funding: Dr. Frangogiannis’s laboratory is supported by NIH grants R01 HL76246 and R01 HL85440.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb Perspect Biol 2016;8:a021873. [Crossref] [PubMed]
- de Larco JE, Todaro GJ. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A 1978;75:4001-5. [Crossref] [PubMed]
- Roberts AB, Lamb LC, Newton DL, et al. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci U S A 1980;77:3494-8. [Crossref] [PubMed]
- Roberts AB, Anzano MA, Lamb LC, et al. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A 1981;78:5339-43. [Crossref] [PubMed]
- Moses HL, Roberts AB, Derynck R. The Discovery and Early Days of TGF-β: A Historical Perspective. Cold Spring Harb Perspect Biol 2016;8:a021865. [Crossref] [PubMed]
- Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol 2011;51:600-6. [Crossref] [PubMed]
- Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev 2010;21:49-59. [Crossref] [PubMed]
- Dickson MC, Martin JS, Cousins FM, et al. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development 1995;121:1845-54. [PubMed]
- Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 1993;90:770-4. [Crossref] [PubMed]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, et al. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 1997;124:2659-70. [PubMed]
- Proetzel G, Pawlowski SA, Wiles MV, et al. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet 1995;11:409-14. [Crossref] [PubMed]
- Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal 2006;8:1907-39. [Crossref] [PubMed]
- Frangogiannis NG. Pathophysiology of Myocardial Infarction. Compr Physiol 2015;5:1841-75. [Crossref] [PubMed]
- Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res 2016;119:91-112. [Crossref] [PubMed]
- Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res 2012;110:159-73. [Crossref] [PubMed]
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 2000;35:569-82. [Crossref] [PubMed]
- Thompson NL, Flanders KC, Smith JM, et al. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol 1989;108:661-9. [Crossref] [PubMed]
- Heine U, Munoz EF, Flanders KC, et al. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol 1987;105:2861-76. [Crossref] [PubMed]
- Bartram U, Molin DG, Wisse LJ, et al. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation 2001;103:2745-52. [Crossref] [PubMed]
- Roberts AB, Roche NS, Winokur TS, et al. Role of transforming growth factor-beta in maintenance of function of cultured neonatal cardiac myocytes. Autocrine action and reversal of damaging effects of interleukin-1. J Clin Invest 1992;90:2056-62. [Crossref] [PubMed]
- Dewald O, Ren G, Duerr GD, et al. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol 2004;164:665-77. [Crossref] [PubMed]
- Wünsch M, Sharma HS, Markert T, et al. In situ localization of transforming growth factor beta 1 in porcine heart: enhanced expression after chronic coronary artery constriction. J Mol Cell Cardiol 1991;23:1051-62. [Crossref] [PubMed]
- Vilahur G, Juan-Babot O, Pena E, et al. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J Mol Cell Cardiol 2011;50:522-33. [Crossref] [PubMed]
- Hao J, Ju H, Zhao S, et al. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol 1999;31:667-78. [Crossref] [PubMed]
- Deten A, Holzl A, Leicht M, et al. Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J Mol Cell Cardiol 2001;33:1191-207. [Crossref] [PubMed]
- Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 2007;74:184-95. [Crossref] [PubMed]
- van Amerongen MJ, Harmsen MC, van Rooijen N, et al. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 2007;170:818-29. [Crossref] [PubMed]
- Dewald O, Zymek P, Winkelmann K, et al. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 2005;96:881-9. [Crossref] [PubMed]
- Meyer A, Wang W, Qu JX, et al. Platelet TGF-beta 1 contributions to plasma TGF-beta 1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood 2012;119:1064-74. [Crossref] [PubMed]
- Saxena A, Dobaczewski M, Rai V, et al. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol 2014;307:H1233-42. [Crossref] [PubMed]
- Dobaczewski M, Xia Y, Bujak M, et al. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol 2010;176:2177-87. [Crossref] [PubMed]
- Weirather J, Hofmann UD, Beyersdorf N, et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 2014;115:55-67. [Crossref] [PubMed]
- Frangogiannis NG, Perrard JL, Mendoza LH, et al. Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation 1998;98:687-98. [Crossref] [PubMed]
- Robertson IB, Rifkin DB. Regulation of the Bioavailability of TGF-β and TGF-β-Related Proteins. Cold Spring Harb Perspect Biol 2016;8:a021907. [Crossref] [PubMed]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 2003;116:217-24. [Crossref] [PubMed]
- Gentry LE, Nash BW. The pro domain of pre-pro-transforming growth factor beta 1 when independently expressed is a functional binding protein for the mature growth factor. Biochemistry 1990;29:6851-7. [Crossref] [PubMed]
- Birdsall HH, Green DM, Trial J, et al. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation 1997;95:684-92. [Crossref] [PubMed]
- Bujak M, Ren G, Kweon HJ., et al. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation 2007;116:2127-38. [Crossref] [PubMed]
- Nishimura SL. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol 2009;175:1362-70. [Crossref] [PubMed]
- Sarrazy V, Koehler A, Chow ML, et al. Integrins alphavbeta5 and alphavbeta3 promote latent TGF-beta1 activation by human cardiac fibroblast contraction. Cardiovasc Res 2014;102:407-17. [Crossref] [PubMed]
- Frangogiannis NG, Ren G, Dewald O, et al. The critical role of endogenous Thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation 2005;111:2935-42. [Crossref] [PubMed]
- Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev 2012;92:635-88. [Crossref] [PubMed]
- Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res 2004;64:24-31. [Crossref] [PubMed]
- Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 2000;11:59-69. [Crossref] [PubMed]
- Kottmann RM, Kulkarni AA, Smolnycki KA, et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-beta. Am J Respir Crit Care Med 2012;186:740-51. [Crossref] [PubMed]
- Lefer AM, Tsao P, Aoki N, et al. Mediation of cardioprotection by transforming growth factor-beta. Science 1990;249:61-4. [Crossref] [PubMed]
- Lefer AM, Ma XL, Weyrich AS, et al. Mechanism of the cardioprotective effect of transforming growth factor beta 1 in feline myocardial ischemia and reperfusion. Proc Natl Acad Sci U S A 1993;90:1018-22. [Crossref] [PubMed]
- Baxter GF, Mocanu MM, Brar BK, et al. Cardioprotective effects of transforming growth factor-beta1 during early reoxygenation or reperfusion are mediated by p42/p44 MAPK. J Cardiovasc Pharmacol 2001;38:930-9. [Crossref] [PubMed]
- Schröder D, Heger J, Piper HM, et al. Angiotensin II stimulates apoptosis via TGF-beta1 signaling in ventricular cardiomyocytes of rat. J Mol Med (Berl) 2006;84:975-83. [Crossref] [PubMed]
- Wenzel S, Henning K, Habbig A, et al. TGF-beta1 improves cardiac performance via up-regulation of laminin receptor 37/67 in adult ventricular cardiomyocytes. Basic Res Cardiol 2010;105:621-9. [Crossref] [PubMed]
- Rainer PP, Hao S, Vanhoutte D, et al. Cardiomyocyte-specific transforming growth factor beta suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circ Res 2014;114:1246-57. [Crossref] [PubMed]
- Travis MA, Sheppard D. TGF-beta activation and function in immunity. Annu Rev Immunol 2014;32:51-82. [Crossref] [PubMed]
- Fava RA, Olsen NJ, Postlethwaite AE, et al. Transforming growth factor beta 1 (TGF-beta 1) induced neutrophil recruitment to synovial tissues: implications for TGF-beta-driven synovial inflammation and hyperplasia. J Exp Med 1991;173:1121-32. [Crossref] [PubMed]
- Wahl SM, Hunt DA, Wakefield LM, et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A 1987;84:5788-92. [Crossref] [PubMed]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol 1998;16:137-61. [Crossref] [PubMed]
- Werner F, Jain MK, Feinberg MW, et al. Transforming growth factor-beta 1 inhibition of macrophage activation is mediated via Smad3. J Biol Chem 2000;275:36653-8. [Crossref] [PubMed]
- Feinberg MW, Shimizu K, Lebedeva M, et al. Essential role for Smad3 in regulating MCP-1 expression and vascular inflammation. Circ Res 2004;94:601-8. [Crossref] [PubMed]
- Kitamura M, Suto T, Yokoo T, et al. Transforming growth factor-beta 1 is the predominant paracrine inhibitor of macrophage cytokine synthesis produced by glomerular mesangial cells. J Immunol 1996;156:2964-71. [PubMed]
- Celada A, Maki RA. Transforming growth factor-beta enhances the M-CSF and GM-CSF-stimulated proliferation of macrophages. J Immunol 1992;148:1102-5. [PubMed]
- Smith WB, Noack L, Khew-Goodall Y, et al. Transforming growth factor-beta 1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J Immunol 1996;157:360-8. [PubMed]
- Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 2000;12:171-81. [Crossref] [PubMed]
- Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol 2015;35:1066-70. [Crossref] [PubMed]
- Ikeuchi M, Tsutsui H, Shiomi T, et al. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res 2004;64:526-35. [Crossref] [PubMed]
- Gonzalez-Quesada C, Cavalera M, Biernacka A, et al. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ Res 2013;113:1331-44. [Crossref] [PubMed]
- Zhang F, Wang H, Wang X, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016;7:52294-52306. [PubMed]
- Ding A, Nathan CF, Graycar J, et al. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol 1990;145:940-4. [PubMed]
- Nelson BJ, Ralph P, Green SJ, et al. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-beta 1. J Immunol 1991;146:1849-57. [PubMed]
- Xiao YQ, Freire-de-Lima CG, Janssen WJ, et al. Oxidants selectively reverse TGF-beta suppression of proinflammatory mediator production. J Immunol 2006;176:1209-17. [Crossref] [PubMed]
- Oh SA, Li MO. TGF-beta: guardian of T cell function. J Immunol 2013;191:3973-9. [Crossref] [PubMed]
- Chen W, Konkel JE. Development of thymic Foxp3(+) regulatory T cells: TGF-beta matters. Eur J Immunol 2015;45:958-65. [Crossref] [PubMed]
- Wahl SM, Swisher J, McCartney-Francis N, et al. TGF-beta: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J Leukoc Biol 2004;76:15-24. [Crossref] [PubMed]
- Hofmann U, Frantz S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res 2015;116:354-67. [Crossref] [PubMed]
- Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors 2011;29:196-202. [Crossref] [PubMed]
- Cucoranu I, Clempus R, Dikalova A, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 2005;97:900-7. [Crossref] [PubMed]
- Eghbali M, Tomek R, Sukhatme VP, et al. Differential effects of transforming growth factor-beta 1 and phorbol myristate acetate on cardiac fibroblasts. Regulation of fibrillar collagen mRNAs and expression of early transcription factors. Circ Res 1991;69:483-90. [Crossref] [PubMed]
- Dobaczewski M, Bujak M, Li N, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res 2010;107:418-28. [Crossref] [PubMed]
- Chua CC, Chua BH, Zhao ZY, et al. Effect of growth factors on collagen metabolism in cultured human heart fibroblasts. Connect Tissue Res 1991;26:271-81. [Crossref] [PubMed]
- Yi X, Li X, Zhou Y, et al. Hepatocyte growth factor regulates the TGF-beta1-induced proliferation, differentiation and secretory function of cardiac fibroblasts. Int J Mol Med 2014;34:381-90. [PubMed]
- Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res 2000;48:89-100. [Crossref] [PubMed]
- Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: a role in inflammation and repair. J Mol Cell Cardiol 2014;70:74-82. [Crossref] [PubMed]
- Cleutjens JP, Verluyten MJ, Smiths JF, et al. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol 1995;147:325-38. [PubMed]
- Kanisicak O, Khalil H, Ivey MJ, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nature communications 2016;7:12260. [Crossref] [PubMed]
- Ruiz-Villalba A, Simon AM, Pogontke C, et al. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol 2015;65:2057-66. [Crossref] [PubMed]
- Frantz S, Hu K, Adamek A, et al. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol 2008;103:485-92. [Crossref] [PubMed]
- Okada H, Takemura G, Kosai K, et al. Postinfarction gene therapy against transforming growth factor-beta signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation 2005;111:2430-7. [Crossref] [PubMed]
- Pinto AR, Ilinykh A, Ivey MJ, et al. Revisiting Cardiac Cellular Composition. Circ Res 2016;118:400-9. [Crossref] [PubMed]
- Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost 2007;97:738-47. [PubMed]
- Kumar AG, Ballantyne CM, Michael LH, et al. Induction of monocyte chemoattractant protein-1 in the small veins of the ischemic and reperfused canine myocardium. Circulation 1997;95:693-700. [Crossref] [PubMed]
- Frangogiannis NG. The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. J Cardiovasc Pharmacol 2014;63:185-95. [Crossref] [PubMed]
- Virag JI, Murry CE. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am J Pathol 2003;163:2433-40. [Crossref] [PubMed]
- Ren G, Michael LH, Entman ML, et al. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem 2002;50:71-9. [Crossref] [PubMed]
- Dobaczewski M, Akrivakis S, Nasser K, et al. Vascular mural cells in healing canine myocardial infarcts. J Histochem Cytochem 2004;52:1019-29. [Crossref] [PubMed]
- Zymek P, Bujak M, Chatila K, et al. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol 2006;48:2315-23. [Crossref] [PubMed]
- Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev 1997;8:21-43. [Crossref] [PubMed]
- Pardali E, ten Dijke P. Transforming growth factor-beta signaling and tumor angiogenesis. Front Biosci (Landmark Ed) 2009;14:4848-61. [Crossref] [PubMed]
- Frangogiannis NG, Mendoza LH, Lewallen M, et al. Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB J 2001;15:1428-30. [PubMed]
- Matsa E, Sallam K, Wu JC. Cardiac stem cell biology: glimpse of the past, present, and future. Circ Res 2014;114:21-7. [Crossref] [PubMed]
- Laflamme MA, Murry CE. Heart regeneration. Nature 2011;473:326-35. [Crossref] [PubMed]
- Massagué J, Xi Q. TGF-β control of stem cell differentiation genes. FEBS Lett 2012;586:1953-8. [Crossref] [PubMed]
- Chablais F, Jazwinska A. The regenerative capacity of the zebrafish heart is dependent on TGFbeta signaling. Development 2012;139:1921-30. [Crossref] [PubMed]
- Behfar A, Zingman LV, Hodgson DM, et al. Stem cell differentiation requires a paracrine pathway in the heart. Faseb J 2002;16:1558-66. [Crossref] [PubMed]
- Li TS, Hayashi M, Ito H, et al. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation 2005;111:2438-45. [Crossref] [PubMed]
- Willems E, Cabral-Teixeira J, Schade D, et al. Small molecule-mediated TGF-beta type II receptor degradation promotes cardiomyogenesis in embryonic stem cells. Cell Stem Cell 2012;11:242-52. [Crossref] [PubMed]
- Chen WP, Liu YH, Ho YJ, et al. Pharmacological inhibition of TGFbeta receptor improves Nkx2.5 cardiomyoblast-mediated regeneration. Cardiovasc Res 2015;105:44-54. [Crossref] [PubMed]
- Massagué J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol 2000;1:169-78. [Crossref] [PubMed]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003;113:685-700. [Crossref] [PubMed]
- Macias MJ, Martin-Malpartida P, Massague J. Structural determinants of Smad function in TGF-beta signaling. Trends Biochem Sci 2015;40:296-308. [Crossref] [PubMed]
- Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol 2005;21:659-93. [Crossref] [PubMed]
- Nurgazieva D, Mickley A, Moganti K, et al. TGF-beta1, but not bone morphogenetic proteins, activates Smad1/5 pathway in primary human macrophages and induces expression of proatherogenic genes. J Immunol 2015;194:709-18. [Crossref] [PubMed]
- Hayashi H, Abdollah S, Qiu Y, et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell 1997;89:1165-73. [Crossref] [PubMed]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577-84. [Crossref] [PubMed]
- Engel ME, McDonnell MA, Law BK, et al. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J Biol Chem 1999;274:37413-20. [Crossref] [PubMed]
- Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. Embo J 2002;21:3749-59. [Crossref] [PubMed]
- Masaki M, Izumi M, Oshima Y, et al. Smad1 protects cardiomyocytes from ischemia-reperfusion injury. Circulation 2005;111:2752-9. [Crossref] [PubMed]
- Zhang D, Gaussin V, Taffet GE, et al. TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med 2000;6:556-63. [Crossref] [PubMed]
- Daniels CE, Wilkes MC, Edens M, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest 2004;114:1308-16. [Crossref] [PubMed]
- Wang S, Wilkes MC, Leof EB, et al. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. Faseb J 2005;19:1-11. [Crossref] [PubMed]
- Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 2010;106:1675-80. [Crossref] [PubMed]
- Panek AN, Posch MG, Alenina N, et al. Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload. PLoS One 2009;4:e6743. [Crossref] [PubMed]
- Gravning J, Orn S, Kaasboll OJ, et al. Myocardial connective tissue growth factor (CCN2/CTGF) attenuates left ventricular remodeling after myocardial infarction. PLoS One 2012;7:e52120. [Crossref] [PubMed]
- Accornero F, van Berlo JH, Correll RN, et al. Genetic Analysis of Connective Tissue Growth Factor as an Effector of Transforming Growth Factor beta Signaling and Cardiac Remodeling. Mol Cell Biol 2015;35:2154-64. [Crossref] [PubMed]
- Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 2014;11:255-65. [Crossref] [PubMed]
- Frangogiannis NG. Targeting the transforming growth factor (TGF)-beta cascade in the remodeling heart: benefits and perils. J Mol Cell Cardiol 2014;76:169-71. [Crossref] [PubMed]
- Tan CK, Tan EH, Luo B, et al. SMAD3 deficiency promotes inflammatory aortic aneurysms in angiotensin II-infused mice via activation of iNOS. J Am Heart Assoc 2013;2:e000269. [Crossref] [PubMed]
- Biernacka A, Cavalera M, Wang J, et al. Smad3 Signaling Promotes Fibrosis While Preserving Cardiac and Aortic Geometry in Obese Diabetic Mice. Circ Heart Fail 2015;8:788-98. [Crossref] [PubMed]
- Regalado ES, Guo DC, Villamizar C, et al. Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res 2011;109:680-6. [Crossref] [PubMed]


