Is early postoperative administration of pregabalin beneficial for patients with lung cancer?—randomized control trial
Introduction
Post-thoracotomy pain syndrome (PTPS) is a well-established, clinically important entity, with persistent pain reported in at 3 and 6 months (1). PTPS has been defined by the International Association for Study of Pain as “Pain that recurs or persists along a thoracotomy scar at least 2 months following a surgical procedure” (2). Several attempts (3) to reduce PTPS, including thoracic epidural, or paravertebral blocks (4), intercostal block (5), cryoanalgesia, as well as administration of nonsteroidal anti-inflammatory drugs (NSAIDs), morphine, ketamine, and gabapentinoids, have been demonstrated. Nevertheless, clinical evidence of prevention of PTPS is lacking. Uncontrolled PTPS can lead to reduced physical function and quality of life as well as to increased healthcare costs.
Video-assisted thoracic surgery (VATS) is applied for almost all types of thoracic surgery because, compared with conventional thoracotomy, it is associated with reduced invasiveness and has equivalent or favourable surgical results (6). Thus, VATS is expected to decrease the prevalence of PTPS. In addition, our research team has evaluated damage to intercostal nerves using the current perception threshold to assess nerve function (7). We confirmed that VATS is a useful procedure to reduce PTPS without using metal retractors that may damage intercostal nerves (7). Nevertheless, we have often encountered patients who, even after VATS, suffer from continuous post-thoracotomy pain, especially around the location of insertion of the chest tube or the access wound. Our research team has also demonstrated damage to intercostal nerves by chest-tube insertion using the same method (8). In addition, Wildgaard and workers (9) investigated 47 patients undergoing VATS. They reported that about 30% of patients had some pain-related functional impairment from the surgical site. Thus, PTPS remains a problem for patients even in the less-invasive era of surgery.
Pregabalin is a derivative of γ-aminobutyric acid. It binds to Ca2+ channels in nerve presynapses and suppresses release of neural transmitters, thereby showing analgesic effects against neuropathic pain (10). Several reports have suggested the efficacy and safety of pregabalin against early post-thoracotomy pain (11-14). However, some reports (15) have not revealed its efficacy or its abuse and dependence (16). Thus, use of pregabalin in the early phase of thoracic surgery is controversial. The purpose of this prospective study was to identify the efficacy of early postoperative administration of pregabalin for surgically treated non-small cell lung cancer (NSCLC) from different viewpoints of pain.
Methods
Study design
The study was a randomized control trial undertaken at Nagasaki University Hospital (Nagasaki, Japan) from January 2014 to December 2015. Approval from the local ethics committee was obtained before study commencement (approval number: 13093038). All patients provided written informed consent before enrolment. This study was registered with the Clinical Trial Registry of the University Hospital Medical Information Network (UMIN 000012386).
Consecutive patients aged 45–75 years scheduled for surgery with primary NSCLC were assessed for study participation. Exclusion criteria were: pre-existing pain; current use of painkillers such as acetaminophen, NSAIDs, pregabalin or gabapentin; contraindication to epidural anaesthesia; chronic renal failure (creatinine clearance <50 mL/min); previous thoracotomy; unstable condition owing to postoperative complications (respiratory failure, re-intubation, re-insertion of chest tube, chemical pleurodesis).
Patients were randomized to the control group or pregabalin group using a random number table by Excel 2010 (Microsoft, Redmond, WA, USA) before surgery. General anaesthesia was induced using propofol and rocuronium. For postoperative pain relief, an epidural catheter was placed according to the site of incision before induction of general anaesthesia. Ropivacaine (8 mg/h) was given for ≤5 days. The epidural catheter was removed simultaneously with the chest tube. All patients received celecoxib (200 mg, p.o., b.d.) for ≥1 week. If good relief from pain was not achieved, a suppository of diclofenac sodium (25 mg) was added without daily limitation (control group). In the pregabalin group, in addition to the analgesia mentioned above, pregabalin (75 mg, p.o., b.d.) was administered from when patients could tolerate oral intake during hospitalization. The diclofenac sodium suppository was added in the same manner of control group.
Preoperative evaluation and surgical method for lung cancer
Routine preoperative staging was chest radiography, computed tomography of the chest, magnetic resonance imaging of the brain, and positron emission tomography/computed tomography. Complete evaluation of cardiac and respiratory functions was done to ensure that patients could tolerate pulmonary resection. Extent of pulmonary resection and systemic dissection of mediastinal or hilar lymph nodes was determined according to clinical stage, performance status, and comorbidities.
VATS procedures were undertaken by visualization through a television monitor only and without metal retractors. Standardized one-access port (4 cm) and three-port placements were used regardless of the resected lobe and segment. Once metal retractors were applied, or if metal retractors were not used but a skin incision >8 cm was made, the procedure was defined as “thoracotomy”. The one chest tube was removed if the total amount of pleural effusion was <250 mL/day, air leakage had not been seen for >24 h, and chylothorax was not apparent.
Outcomes
Primary endpoint was the frequency of additional usage of a suppository of diclofenac sodium (25 mg) during hospitalization. Secondary endpoints were intensity of ongoing pain, frequency of neuropathic pain, and pain catastrophizing in both groups. We also recorded: patient characteristics; age; sex; body mass index (BMI); type of pulmonary resection (lobectomy/limited); type of thoracotomy (open: using metal retractors/VATS); type of lymph-node dissection (radical/limited); operation time; bleeding; duration of chest-tube insertion; postoperative stay in hospital.
Ongoing pain
Intensity of ongoing pain was demonstrated by a Numeric Rating Scale (NRS) (0: no pain; −10: worst possible pain). The NRS score was obtained 3 times a day from nurses blinded to the study protocol. The worst NRS score during the day was used in the present study.
Neuropathic pain
Evaluation of neuropathic pain was conducted by the painDETECT questionnaire (PDQ), a screening tool for neuropathic pain devised by Freynhagen and colleagues (17). This questionnaire was translated into Japanese and its validity and reliability evaluated by Matsubayashi and workers (18). PDQ characteristics are shown elsewhere (17,18) but comprise three components. Main component is “pain gradation”, and the patient is asked to identify eight pathologic pain sensations: “burning”, “tingling”, or “pricking” sensations, “tactile” and “thermal” allodynia, “electric shock-like” sensations, “numbness”, and “pressure-evoked pain” sensation (18). The patient grades each type of pain as 0= “none”, 1= “hardly noticed”, 2= “slightly”, 3= “moderately”, 4= “strongly”, or 5= “very strongly”. Thus, this main component of PDQ yields scores from 0 to 35 points. Second component is termed “pattern of pain course”. The patient grades each type of pain as 0= “persistent pain with slight fluctuations”, −1= “persistent pain with pain attacks”, 1= “pain attacks without pain between them”, and 1= “pain attacks with pain between them”. Third component is termed “radiating pain”. The patient grades “2” or “0” as “pain that radiates to other regions of their body”. Thus, the PDQ score is calculated by addition of the score of the three components (maximum possible score =38, minimum possible score =−1).
Pain catastrophizing
Evaluation of pain catastrophizing was conducted using the Pain Catastrophizing Scale (PCS), a screening tool for pain catastrophizing devised by Sullivan and colleagues (19). This questionnaire was translated into Japanese and its validity and reliability evaluated by Matsuoka and workers (20). PCS characteristics are shown elsewhere (19,21), but the PCS is a 13-item questionnaire related to three catastrophizing components when subjects are in pain: “rumination”, “magnification”, and “helplessness”. Rating is made on a five-point scale from 0= “not at all” to 4= “all the time”. Scores range from 0 (“no catastrophizing”) to 52 (“severe catastrophizing”) with no clear cutoff score used to distinguish “high” from “low” catastrophizing (21).
Follow-up
NRS was obtained prior to and every day following surgery and 1 and 3 months after discharged. PDQ was employed on postoperative day 7 (POD7) as well as 1 and 3 months after discharged. PCS was scored before surgery and on POD7 as well as 1 and 3 months after discharged. Patients visited outpatient clinics 1 and 3 months after discharged. If this schedule was not possible then questionnaire was posted to their home.
Statistical analyses
The sample size was calculated based on how many times pregabalin administration could reduce the frequency use of additional NSAIDs. In our experience, 70% of surgical patients need a NSAID twice and 30% need it once. With regard to pregabalin use, it was anticipated that 70% of patients would need it once and 30% would need it twice. Sample sizes of 29 in the control group and 29 in the pregabalin group could achieve 80% power to detect a difference between the groups of 40%. The test statistic used was the two-sided Fisher’s exact test and P<0.05 considered significant. Considering a dropout of 20%, we concluded that the target sample size was 74 patients.
Patients who underwent randomization and completed the study protocol were included in the analyses. Patient characteristics were compared between the control group and pregabalin group. Data are described as frequencies for categorical variables and as the median and interquartile range (IQR) for quantitative variables. Associations between variables were assessed with the Fisher’s exact test for categorical variables and Wilcoxon’s rank-sum test for quantitative variables.
A P value of 0.05 or less (two-sided) was considered to indicate statistical significance. JMP v11 (SAS Institute, Cary, NC, USA) was used for statistical analyses.
Results
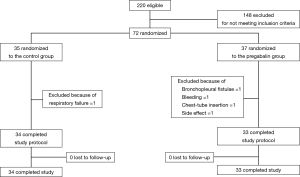
A total of 220 cases of NSCLC surgery were undertaken from December 2013 to December 2015. A total of 148 cases were excluded for not meeting inclusion criteria. Of 72 cases, 35 patients were randomized to the control group and 37 were in the pregabalin group. In the control group, one patient was excluded owing to postoperative respiratory failure. In the pregabalin group, four cases were excluded owing to bronchopleural fistulae after right middle lobectomy and fatal bleeding after right upper lobectomy; both resulted in hospital mortality, re-insertion of a chest tube, and dizziness that seemed to be an adverse effect of pregabalin. Finally, 34 patients in the control group and 33 in the pregabalin group completed the study and were assessed (Figure 1).
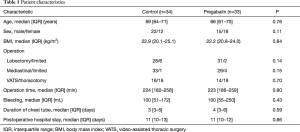
Table 1 shows patient characteristics. There were no significant differences between groups in terms of age (P=0.76), BMI (P=0.84), type of pulmonary resection (lobectomy/limited; P=0.14), type of thoracotomy (open/VATS; P=0.70), type of lymph-node dissection (radical/limited; P=0.15), operation time (P=0.90), bleeding (P=0.43), duration of chest-tube insertion (P=0.59) or duration of postoperative stay in hospital (P=0.86).
Full table
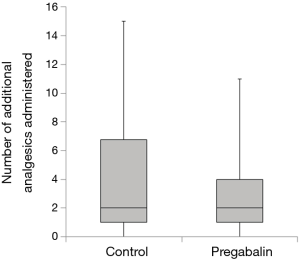
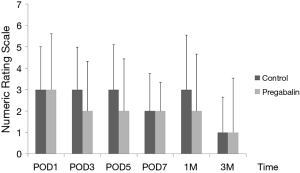
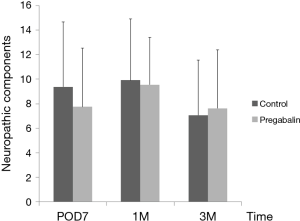
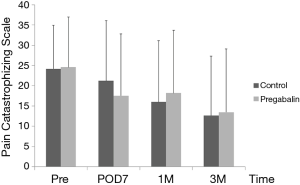
Median number of additional NSAID analgesics used between the control group and pregabalin group was not significant [n=2 suppositories (IQR, 1.0–6.8), n=2 suppositories (IQR, 1.0–4.0), P=0.78] (Figure 2). No significant difference between NRS scores in the control group and pregabalin group was recorded, respectively: 3 and 3 on POD1 (P=0.72); 3 and 2 on POD3 (P=0.92); 3 and 2 on POD5 (P=0.77); 2 and 2 on POD7 (P=0.28); 3 and 2 at 1 month after surgery (P=0.99); 1 and 1 at 3 months after surgery (P=0.13) (Figure 3). No significant difference between PDQ scores in the control group and pregabalin group was recorded, respectively: 9.4±5.4 and 7.8±4.9 on POD7 (P=0.20); 9.9±5.1 and 9.5±3.9 at 1 month after surgery (P=0.77); 7.1±4.6 and 7.6±4.8 at 3 months (P=0.65) (Figure 4). A wide range of PDQ scores (0 to 28) was observed. No significant difference between PCS scores in the control group and pregabalin group was recorded, respectively: 24.2±10.9 and 24.6±12.6 before surgery (P=0.89); 21.2±15.1 and 17.6±15.5 on POD7 (P=0.34); 16.0±15.4 and 18.2±15.7 at 1 month after surgery (P=0.57); 12.6±14.9 and 13.5±15.9 at 3 months after surgery (P=0.83) (Figure 5).
Discussion
This randomized trial was based on the data of 72 patients who underwent pulmonary resection for NSCLC. We found that early administration of pregabalin was not beneficial in terms of reducing the intensity of ongoing pain, frequency of neuropathic pain, and PCS.
Pregabalin has been shown to elicit analgesic effects and is noted for its efficacy and safety against early post-thoracotomy pain (11-14). Moreover, the action of antidepressants has been reported to reduce anxiety and sleep quality in surgical patients, as well as to reduce pain and analgesic consumption (22). Identification and reduction of psychological factors can also help to optimize pain management in thoracic surgery. However, some reports (15) have not revealed the efficacy of pregabalin against neuropathic pain and raised the problem of its abuse and dependence (16). Thus, we wished to identify the efficacy of early postoperative administration of pregabalin for surgically treated patients with NSCLC from different viewpoints of pain expression.
In the present study, the NRS score decreased gradually after surgery, but post-thoracotomy pain was present 3 months after surgery. This finding was compatible with data from our previous report (7) and other reports (1,3,9,23). Early administration of pregabalin did not contribute to reducing acute and chronic pain compared with standard analgesia. We assumed that the reason was pain has been assessed subjectively by means of verbal/visual intensity scales and questionnaires. NRS is also a subjective assessment tool, and pain itself in each patient can be ambiguous. However, the way of pain is expressed can be affected by ethnicity, sex, age, and other factors. Using only NRS cannot be used to evaluate post-thoracotomy pain. In addition, the NRS score was relatively low immediately after surgery, thus a significant difference between the two groups could not been observed.
For screening of neuropathic pain, two main cutoff points for scores have been found to be appropriate: <12 denotes that a neuropathic component is unlikely (<15%); score ≥19 denotes that a neuropathic component is likely (>90%) (17). In our study, 4 (11.8%) of 34 subjects in the control group and 3 (9.1%) of 33 cases in the pregabalin group had a PDQ score ≥19, which were lower scores than we expected for neuropathic components during this period. For further evaluation, among patients with a high PDQ score (≥13) (n=10 in the pregabalin group and n=14 in the control group), no significant reduction of the PDQ score was found even in patients with possible neuropathic components. Thus, in the pain experienced in the early postoperative period, nociceptive pain (not neuropathic pain) comprised most of the post-thoracotomy pain. Steegers and colleagues (23) reported that only half of the chronic pain after thoracic surgery showed a neuropathic component, and that more extensive surgery and pleurectomy were predictive factors. They suggested that a visceral component was more important than nerve injury, and that the cause of neuropathic pain was not simply damage to a single nerve but involved multifactorial mechanisms. This phenomenon was the reason for lower scores for neuropathic components in contrast to NRS. We speculate that the daily regimen for pain management that we use (including epidural analgesia) might be effective, and that the efficacy of PDQ for thoracic surgery has not been established due to the limited number of reports that have used it.
Cognitive and psychological factors have been reported to have important roles in the severity of postoperative pain (9,20,21,24). High levels of pain catastrophizing are associated with heightened pain experience and appear to contribute to the development of chronic pain (24). However, Wildgaard and colleagues (9) reported that although pain-related functional impairment after VATS was lower, no psychological factors could be used to predict persistent postoperative pain. In addition, Khan and workers (24) reviewed pain catastrophizing and its association with postoperative pain and quality of life. They reported that there was no consensus on the relationship between pain catastrophizing and analgesic consumption. Data from those reports are compatible with our results in that the efficacy of pregabalin against pain catastrophizing could not be demonstrated, thereby making it difficult for clinicians to reduce PTPS using psychological intervention during perioperative periods.
Results from our study suggest that we should not try early administration of pregabalin for patients with fewer neuropathic-pain components but that we should limit it to patients with obvious neuropathic components, and administer the appropriate dose of pregabalin.
The present study had three main limitations. First, the study cohort was small and obtained from a single institution. Second, VATS patients and thoracotomy patients were included in both groups, thereby hampering evaluation. We should have limited our assessment to thoracotomy patients. Finally, the pregabalin dose was set 150 mg per day and we did not allow dose increases because we wished to avoid adverse effects such as dizziness and drowsiness. As a result of this rigid limitation of dose, only 1 (1.4%) patient withdrew from the trial owing to dizziness, and this clinical trial could be completed. Future studies are needed to address these limitations and to reduce PTPS.
In conclusion, early postoperative administration of pregabalin was not beneficial for reducing the NRS score or neuropathic pain or improving pain catastrophizing in patients suffering from NSCLC.
Acknowledgements
We thank Dr. Masahiko Sumitani (Anesthesiology and Pain Relief Center, The University of Tokyo Hospital) for allowing us to use the Japanese version of the painDETECT questionnaire and Prof. Yuji Sakano (Department of Psychological Science, Health Sciences University of Hokkaido) for allowing us to use the Japanese version of the Pain Catastrophizing Scale. This study was funded by a research grant in 2015 from the Nagasakiken Medical Association.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval from the local ethics committee was obtained before study commencement (approval number: 13093038). All patients provided written informed consent before enrolment.
References
- Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain 2014;15:887-97. [Crossref] [PubMed]
- Merskey H, Bogduk H. Classification of chronic pain. In: Merskey H, Bogduk H. editors. Descriptions of chronic pain syndromes and definitions of pain terms. 2nd edition. Seattle, WA: ISAP Press, 1994:143-4.
- Rodriguez-Aldrete D, Candiotti KA, Janakiraman R, et al. Trends and New Evidence in the Management of Acute and Chronic Post-Thoracotomy Pain-An Overview of the Literature from 2005 to 2015. J Cardiothorac Vasc Anesth 2016;30:762-72. [Crossref] [PubMed]
- Matyal R, Montealegre-Gallegos M, Shnider M, et al. Preemptive ultrasound-guided paravertebral block and immediate postoperative lung function. Gen Thorac Cardiovasc Surg 2015;63:43-8. [Crossref] [PubMed]
- Khalil KG, Boutrous ML, Irani AD, et al. Operative Intercostal Nerve Blocks With Long-Acting Bupivacaine Liposome for Pain Control After Thoracotomy. Ann Thorac Surg 2015;100:2013-8. [Crossref] [PubMed]
- Cajipe MD, Chu D, Bakaeen FG, et al. Video-assisted thoracoscopic lobectomy is associated with better perioperative outcomes than open lobectomy in a veteran population. Am J Surg 2012;204:607-12. [Crossref] [PubMed]
- Miyazaki T, Sakai T, Tsuchiya T, et al. Assessment and follow-up of intercostal nerve damage after video-assisted thoracic surgery. Eur J Cardiothorac Surg 2011;39:1033-9. [Crossref] [PubMed]
- Miyazaki T, Sakai T, Yamasaki N, et al. Chest tube insertion is one important factor leading to intercostal nerve impairment in thoracic surgery. Gen Thorac Cardiovasc Surg 2014;62:58-63. [Crossref] [PubMed]
- Wildgaard K, Ringsted TK, Hansen HJ, et al. Persistent postsurgical pain after video-assisted thoracic surgery--an observational study. Acta Anaesthesiol Scand 2016;60:650-8. [Crossref] [PubMed]
- Dooley DJ, Donovan CM, Meder WP, et al. Preferential action of gabapentin and pregabalin at P/Q-type voltage-sensitive calcium channels: inhibition of K+-evoked [3H]-norepinephrine release from rat neocortical slices. Synapse 2002;45:171-90. [Crossref] [PubMed]
- Yoshimura N, Iida H, Takenaka M, et al. Effect of Postoperative Administration of Pregabalin for Post-thoracotomy Pain: A Randomized Study. J Cardiothorac Vasc Anesth 2015;29:1567-72. [Crossref] [PubMed]
- Matsutani N, Dejima H, Takahashi Y, et al. Pregabalin reduces post-surgical pain after thoracotomy: a prospective, randomized, controlled trial. Surg Today 2015;45:1411-6. [Crossref] [PubMed]
- Imai Y, Imai K, Kimura T, et al. Evaluation of postoperative pregabalin for attenuation of postoperative shoulder pain after thoracotomy in patients with lung cancer, a preliminary result. Gen Thorac Cardiovasc Surg 2015;63:99-104. [Crossref] [PubMed]
- Mishra A, Nar AS, Bawa A, et al. Pregabalin in Chronic Post-thoracotomy Pain. J Clin Diagn Res 2013;7:1659-61. [PubMed]
- Brulotte V, Ruel MM, Lafontaine E, et al. Impact of pregabalin on the occurrence of postthoracotomy pain syndrome: a randomized trial. Reg Anesth Pain Med 2015;40:262-9. [Crossref] [PubMed]
- Gahr M, Freudenmann RW, Hiemke C, et al. Pregabalin abuse and dependence in Germany: results from a database query. Eur J Clin Pharmacol 2013;69:1335-42. [Crossref] [PubMed]
- Freynhagen R, Baron R, Gockel U, et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911-20. [Crossref] [PubMed]
- Matsubayashi Y, Takeshita K, Sumitani M, et al. Validity and reliability of the Japanese version of the painDETECT questionnaire: a multicenter observational study. PLoS One 2013;8:e68013. [Crossref] [PubMed]
- Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess 1995;7:524-32. [Crossref]
- Matsuoka H, Sakano Y. Assessment of cognitive aspect of pain: development, reliability, and validation of Japanese version of Pain Catastrophizing Scale. Jpn J Psychosom Med 2007;47:95-102.
- Khan RS, Skapinakis P, Ahmed K, et al. The association between preoperative pain catastrophizing and postoperative pain intensity in cardiac surgery patients. Pain Med 2012;13:820-7. [Crossref] [PubMed]
- Shimony N, Amit U, Minz B, et al. Perioperative pregabalin for reducing pain, analgesic consumption, and anxiety and enhancing sleep quality in elective neurosurgical patients: a prospective, randomized, double-blind, and controlled clinical study. J Neurosurg 2016;125:1513-22. [Crossref] [PubMed]
- Steegers MA, Snik DM, Verhagen AF, et al. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain 2008;9:955-61. [Crossref] [PubMed]
- Khan RS, Ahmed K, Blakeway E, et al. Catastrophizing: a predictive factor for postoperative pain. Am J Surg 2011;201:122-31. [Crossref] [PubMed]