Duration of antibiotic therapy in the intensive care unit
Introduction
Antimicrobial stewardship (AMS) programs are those that aim to educate and encourage evidence-based antimicrobial prescribing practices in order to stem antibiotic overuse, and thus antimicrobial resistance. Antibiotic resistance has become a grave concern globally with particular concern for the misuse or overuse of antibiotics, a phenomenon that has led to the emergence of multidrug resistant (MDR) bacterial pathogens. There are certain well defined clinical situations where prolonged therapy is beneficial and indicated such as for treatment of infective endocarditis, osteomyelitis, fungal infections or Legionella pneumonia, but prolonged duration of antibiotic therapy is associated with increased resistance, medicalising effects, high costs and adverse drug reactions (1). Hence one of the key principles of AMS is to decrease unnecessary and prolonged antibiotic administration to reduce antibiotic pressure and prevent the emergence of MDR pathogens. On one hand, “time is life”, as there is a clear association between the timing of antibiotic administration and the mortality in patients presenting septic shock (2). Therefore under such conditions, early empiric broad-spectrum therapy in the intensive care environment is justified and saves lives. On the other hand, there is a growing evidence base that reduction in the length of antibiotic courses can minimize the consequences of antibiotic overuse in critical care, including antibiotic resistance, adverse effects, collateral damage and costs. The present review aims to summarize, with the use of key questions in daily clinical practice, recommendations on antibiotic administration in critically ill patients with severe infections.
Is 2 weeks (or longer) of antibiotic therapy justified in critically ill patients?
Ventilator-associated pneumonia (VAP) is the most common and most serious hospital-acquired infection reported among patients receiving mechanical ventilation (3). The optimal duration of antimicrobial treatment for VAP is unknown and it is possible that there is no ‘one size fits all’ approach. However, Dennesen et al. demonstrated that VAP resolution of clinical parameters with adequate antibiotic therapy occurs during the first 6 days of treatment—the PaO2/FIO2 ratio is the most specific marker—and continuing therapy beyond this can be deleterious and it was independent of microbiologic sterilization (4). Recent systematic reviews also suggest that shorter therapy is safe and significantly increases antibiotic-free days (5,6). Pugh et al. reviewed eight randomized trials concerning VAP/HAP and compared a short seven to 8-day antibiotic course with a prolonged ten to 15-day course. They found an increase in antibiotic-free days at day 28 and a reduction in recurrent VAP infections due to MDR pathogens. There were no differences in mortality, recurrent pneumonia or in treatment failure. Dimopoulos et al. similarly found that short-course (7–8 days) therapy compared to long-course regimens of 10–15 days increased antibiotic-free days without differences in mortality, recurrent pneumonia, and ventilator-free days. Along with these findings the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) now recommend a 7-day course of antimicrobial for patients with VAP (strong recommendation) (7).
Should we use biomarkers to stop antibiotic therapy in critically ill patients?
Clinical signs of infection can be difficult to interpret especially in the critical care environment. Cultures may only be positive approximately 10% of patients with pneumonia (8) and even scoring systems like the clinical pulmonary infection score (CPIS) may not be able to identify patients who can safely have their antibiotics discontinued (1,9). In the context of diagnostic uncertainty, surrogate biomarkers are useful to estimate the presence of a bacterial infection.
Among the various biomarkers used in clinical practice, C-reactive protein (CRP) and procalcitonin (PCT) are the most widely used in critical care setting. PCT levels correlate well with several systemic inflammatory conditions, bacterial infec tions, are a marker of disease severity, prognosis and decrease upon recovery (10,11), therefore PCT may be helpful to decide which patients are responding to therapy and in whom antibiotic treatment can be ceased. The landmark PRORATA trial conducted in medical patients admitted to intensive care unit (ICU) showed that a PCT driven algorithm was non-inferior to standard care, but significantly reduced antibiotic exposure (12). This study was followed by a Cochrane meta-analysis by Schuetz et al., which included 14 trials with 4,221 patients with respiratory infection. PCT guidance was not associated with increased mortality or treatment failure, but the total antibiotic exposure was significantly lower than in non-PCT algorithms. Interestingly, patient included were immunocompetent and some pathogens were excluded (i.e., Legionella or Pseudomonas infections) and in the subgroup of VAP the PCT based care reduced the antibiotic treatment period only to 11 days from 14 (13), which is considerably longer than currently recommended (7).
Among the other cytokines involved in the inflammatory response, a significant rise in interleukin 6 (IL-6) (P<0.001), CRP (P<0.001), PCT (P=0.004), and interleukin 8 (IL-8) (P=0.02) levels on day 1 of hospitalization predicted early treatment failure in patients with community-acquired pneumonia (CAP) (10). The best correlation was with IL-6, however it is not routinely measured currently. It is important to note that blood levels of CRP are primarily regulated by the production of IL-6 and therefore in clinical practice the use of CRP can be seen as a surrogate. A recent randomised trail also found that CRP might be as useful as PCT in reducing antibiotic use in a predominantly medical population of septic patients (14).
To distinguish bacterial infection vs. inflammation in a surgical subgroup is somewhat more difficult than in a medical cohort and the normally occurring post-operative systematic inflammatory reaction makes biomarkers levels difficult to interpret. PCT thresholds to differentiate bacterial infection from inflammation are commonly higher in perioperative medicine than in medical patients (15). A frequently accepted PCT threshold of 0.5 ng/mL or a drop from the peak of at least 80% failed to predict, in a single centre observational study, treatment response in a subpopulation of septic shock patients admitted for an intra-abdominal infection. Although 95% of patients in whom PCT decreased below 0.5 ng/mL responded successfully to treatment, 50% of the patients in whom PCT remained superior to 0.5 ng/mL also responded positively to treatment making a threshold of 0.5 ng/mL specific but not sensitive (16). A systematic review and meta-analysis also concluded that PCT cannot reliably differentiate infectious from non-infectious causes of inflammation in critically ill patients (17).
Our recommendation is that biomarkers do have a valuable role in helping guide antibiotic duration but should be interpreted cautiously in the context of the clinical situation. They can guide and assist clinicians in difficult scenarios, but should never substitute a physician’s clinical decision to discontinue antibiotics in the intensive care setting.
Would longer antibiotic course reduce recurrences?
One of the main advantages of shorter courses of antibiotic therapy is to reduce selective pressure for MDR pathogens and therefore prevent the emergence of antibiotic resistance. Data published by Micek et al. suggest that shorter courses of empiric antibiotic treatment can be safely prescribed to patients with suspected VAP based on the occurrence of a second episode of VAP, which was similar in both groups (18). Chastre et al., in a randomized control trial (RCT) that compared two antibiotic regimens for patients with VAP (8 vs. 15 days), were even able to demonstrate that among patients who had received 8 days of antibiotics, recurrent pulmonary infections with MDR pathogens emerged significantly less frequently than in those with longer antibiotic courses (42.1% vs. 62.3% of recurrent infections; P=0.04) (19).
Non-fermenting Gram-negative bacilli (NF-GNB) like Acinetobacter spp. similar to Pseudomonas spp. create a biofilm, have a high level of intrinsic resistance and have ample ways to develop and horizontally transfer resistance. Nosocomial pneumonias caused by these bacteria are difficult to treat and they often colonise the respiratory tract especially if a patient is intubated and ventilated. Rello et al. using molecular biotyping, showed that recurrent episodes of VAP caused by Pseudomonas aeruginosa were frequently caused by persistence of the bacteria in the respiratory tract (20). An elegant double blind prospective trial by Kollef et al. demonstrated that among patients with microbiologically confirmed VAP, a fixed 7-day antibiotic course had non-significant higher rates of clinical failure and mortality compared to a fixed 10-day course. Moreover, patients with VAP attributed to P. aeruginosa had a statistically greater risk of 28-day all-cause mortality. Even though they compared two different antibiotics from same group they concluded that a shorter treatment course played a significant role in this survival difference (21). A subsequent Cochrane meta-analysis did not find mortality or other clinical differences in subgroup of patients with VAP due to a NF-GNB, including P. aeruginosa and A. baumannii. Nevertheless, the short course of antibiotics was associated with higher recurrence rate (41.8% vs. 24.7%; OR, 2.18; 95% CI, 1.14–4.16) (22), therefore, a week of treatment in pneumonia cases caused by NF-GNB may not be enough and the integration of biomarkers, clinical judgment, and microbiologic eradication should be integrated in global decision making process (23).
Does prolonged antibiotic treatment help in clinical cure and sterilization?
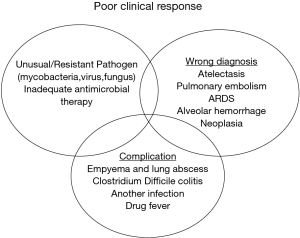
Endotracheal colonization is common in critical care and represents a continuum to VAP. Colonisation however is not equivalent to infection of distal airways and bacterial eradication from endotracheal aspirates is a poor marker for clinical response (Figure 1). A study of patients receiving prolonged mechanical ventilation without clinical evidence of pneumonia found a high burden of bacteria within the distal airway without clinical evidence of VAP (24). Appropriate antimicrobial therapy can rapidly eradicate colonization by S. pneumoniae, H. influenzae, and S. aureus, but fails to eradicate Enterobacteriaceae and P. aeruginosa. This suggests that follow-up of this parameter is an unreliable marker of treatment success when these pathogens are involved, particularly if intensive care treatment is prolonged as tracheal colonization with resistant pathogens frequently occurs during the second week of therapy (4). Therefore microbiological criteria alone are not reliable and should not be used to justify a prolonged antibiotic course, as clinical cure does not equate to microbiological eradication.
Is antibiotic therapy an innocent and always beneficial therapy particularly if prolonged?
There have been multiple studies suggesting a correlation between prolonged antibiotic therapy and an increase in MDR pathogens. These infections are associated with an increased consumption of healthcare resources and mortality (25,26). Strategies minimising the use of broad-spectrum antibiotics and ensuring prompt antibiotic administration should be adopted in order to reduce antibiotic resistance rates. The decision to stop inappropriate antibiotic therapy is difficult in the critical care environment. A study by Singh found that,
- Most antibiotic use in the ICU, in fact, occurs in patients in whom pulmonary infiltrates are not caused by pneumonia but by pulmonary oedema or atelectasis;
- Antibiotics once initiated, were invariably continued for 4 to 20 days (average 10 days), regardless of the likelihood of pneumonia;
- The conventional approach, where the antibiotic course was prolonged, was associated with significantly higher rate MDR pathogens (27). Population-level analysis, using mathematical modelling also suggests that it is the very use of antibiotics that drives the transmission dynamics of antimicrobial-resistant bacteria. In the absence of treatment, and selective antimicrobial pressure, the wild-type non-resistant bacteria have a selective advantage over the resistant strain. During treatment, however, the resistant bacteria gain the advantage over the non-resistant strain (28).
What strategies do we use to make the decision to discontinue antibiotic therapy in critically ill patients?
In order to use appropriate therapy in a timely manner, without overusing antibiotics, the ATS/IDSA guidelines for patients with suspected HAP/VAP recommend using clinical criteria when initiating early antibiotics after collection of cultures (7). We recommend that empiric antibiotics thereafter may need to be modified once the results of blood or respiratory tract cultures become available. Modification may also be necessary if a resistant or unsuspected pathogen is found in a patient developing treatment failure. Alternatively, therapy can be de-escalated or narrowed if an anticipated organism (such as P. aeruginosa or an Acinetobacter species) was not found or if the organism isolated is sensitive to a less broad-spectrum antibiotic than was used in the initial regimen (Figure 2). Clinical improvement is good indicator of therapy response, however as Luna et al. suggested, most of the traditional measures of infection such as radiographic infiltrate, amount and quality of secretions, fever, and high white cell counts (WCC) are poor predictors of clinical response to therapy, whereas a more specific physiologic variable, the PaO2/FiO2 ratio, can much more accurately differentiate between responders and non-responders (9).
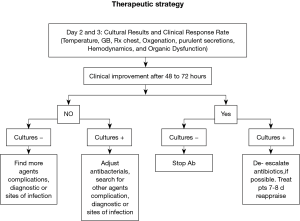
Another way to reduce inappropriate antibiotic exposure is to do invasive diagnostic testing including quantitative culture of protected specimen brush (PSB) samples or bronchoalveolar lavage (BAL) samples. Fagon et al. found that such an approach compared to clinical criteria alone significantly reduced antimicrobial exposure (29). However such invasive diagnostic methods are currently not recommended as their use has failed to improve clinical outcome. It is important to stress that, if BAL or PSB are performed and quantitative culture results are below the diagnostic threshold for VAP, antibiotics should be withheld rather than continued (7).
It is also important to mention that even though Candida is frequently cultured (rates can be as high as 60% in the ICU population) from the respiratory tract, in immunocompetent patients Candida pneumonia is a non-existent clinical entity based on a well-conducted large autopsy studies (30). These patient had higher severity scores and organ dysfunction at admission and at the onset of pneumonia, but isolation of Candida spp. from the respiratory tract does not influence outcomes in these patients, regardless of the use or not of antifungal therapy (31). Therefore we recommend not starting antifungal treatment for patients with Candida spp. isolated in respiratory samples. Isolation, whether using invasive or non-invasive diagnostic methods, reflects Candida spp. colonisation rather than infection and should not be treated.
Is there a solution for every clinical situation?
A systematic review by Havey et al. concluded that there are other clinical scenarios like blood stream infection, pyelonephritis, soft tissue infection and even abdominal infection, where shorter than traditional antibiotic courses in selected cases may be safe and effective (32). We consider that, in general, prolonged antibiotic use is neither beneficial nor results in better patient outcomes. However, we would like to highlight that AMS programs are beneficial. At this time the optimal metrics to benchmark antimicrobial use are still contentious and “appropriateness of use” is probably the most controversial term. Appropriate use depends on the local antimicrobial resistance profile and therefore has different ecological answers. Merely the “amount” of antibiotics used is not a straightforward metric for appropriateness. Another important point is to correctly implement trials meant to be done in “critically ill patients” to truly “critically ill patients”. For instance, a randomized Study to Optimize Peritoneal Infection Therapy (STOP-IT) trial compared two strategies guiding the duration of antimicrobial therapy for the management of complicated intra-abdominal infection. Sawyer et al. found that the outcomes after fixed-duration antibiotic therapy (approximately 4 days) were similar to those after a longer course of antibiotics (approximately 8 days). Whilst, we consider this a very well designed study, the authors highlighted that this strategy showed non-inferior outcomes in patients who had undergone an adequate source-control procedure. It is important to note when interpreting current literature if the study focuses on the critically ill population. Clearly this study cannot be applied to critically ill patients as in this population; the mortality rate was very low (≈1%). Therefore, our recommendation is to interpret the clinical situation in context before implementing the study results in critically ill patients. Many studies despite using “severe”, “complicated”… do not truly reflect critically ill patients.
Summary
Antibiotic resistance is a serious problem all over the world and antibiotics have become less effective or, in some cases ineffective, resulting in a global health security emergency. We consider that the best way to decrease antibiotic duration is both to stop antibiotics when not needed (sterile invasive cultures with clinical improvement) and not to start antibiotics when not indicated (treating colonization). There are multiple ways to identify appropriate therapy length although none are perfect. Our recommendation is shortening the antibiotic course as an effective and safe way to decrease inappropriate antibiotic exposure. However, we do not recommend a ‘one size fits all’ approach and some clinical situations, including infection with NG-GNB, inadequate source control, infective endocarditis, osteomyelitis, fungal infections or Legionella pneumonia represent difficult to treat infections and warrant case-by-case evaluation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lawrence KL, Kollef MH. Antimicrobial stewardship in the intensive care unit: advances and obstacles. Am J Respir Crit Care Med 2009;179:434-8. [Crossref] [PubMed]
- Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 2011;39:2066-71. [Crossref] [PubMed]
- Vincent JL, Bihari DJ, Suter PM, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA 1995;274:639-44. [Crossref] [PubMed]
- Dennesen PJ, van der Ven AJ, Kessels AG, et al. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med 2001;163:1371-5. [Crossref] [PubMed]
- Pugh R, Grant C, Cooke RP, et al. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev 2011.CD007577. [PubMed]
- Dimopoulos G, Poulakou G, Pneumatikos IA, et al. Short- vs long-duration antibiotic regimens for ventilator-associated pneumonia: a systematic review and meta-analysis. Chest 2013;144:1759-67. [Crossref] [PubMed]
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111. [Crossref] [PubMed]
- Garcia-Vidal C, Carratalà J. Early and late treatment failure in community-acquired pneumonia. Semin Respir Crit Care Med 2009;30:154-60. [Crossref] [PubMed]
- Luna CM, Blanzaco D, Niederman MS, et al. Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med 2003;31:676-82. [Crossref] [PubMed]
- Martin-Loeches I, Valles X, Menendez R, et al. Predicting treatment failure in patients with community acquired pneumonia: a case-control study. Respir Res 2014;15:75. [PubMed]
- Jensen JU, Heslet L, Jensen TH, et al. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med 2006;34:2596-602. [Crossref] [PubMed]
- Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375:463-74. [Crossref] [PubMed]
- Schuetz P, Müller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012.CD007498. [PubMed]
- Oliveira CF, Botoni FA, Oliveira CR, et al. Procalcitonin versus C-reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Crit Care Med 2013;41:2336-43. [Crossref] [PubMed]
- Clec'h C, Fosse JP, Karoubi P, et al. Differential diagnostic value of procalcitonin in surgical and medical patients with septic shock. Crit Care Med 2006;34:102-7. [Crossref] [PubMed]
- Jung B, Molinari N, Nasri M, et al. Procalcitonin biomarker kinetics fails to predict treatment response in perioperative abdominal infection with septic shock. Crit Care 2013;17:R255. [Crossref] [PubMed]
- Tang BM, Eslick GD, Craig JC, et al. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 2007;7:210-7. [Crossref] [PubMed]
- Micek ST, Ward S, Fraser VJ, et al. A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator-associated pneumonia. Chest 2004;125:1791-9. [Crossref] [PubMed]
- Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 2003;290:2588-98. [Crossref] [PubMed]
- Rello J, Mariscal D, March F, et al. Recurrent Pseudomonas aeruginosa pneumonia in ventilated patients: relapse or reinfection? Am J Respir Crit Care Med 1998;157:912-6. [Crossref] [PubMed]
- Kollef MH, Chastre J, Clavel M, et al. A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit Care 2012;16:R218. [Crossref] [PubMed]
- Pugh R, Grant C, Cooke RP, et al. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev 2015.CD007577. [PubMed]
- Martin-Loeches I, Torres A. A week seems to be weak: tailoring duration of antibiotic treatment in Gram-negative ventilator-associated pneumonia. Crit Care 2013;17:106. [Crossref] [PubMed]
- Baram D, Hulse G, Palmer LB. Stable patients receiving prolonged mechanical ventilation have a high alveolar burden of bacteria. Chest 2005;127:1353-7. [PubMed]
- Zilahi G, Artigas A, Martin-Loeches I. What's new in multidrug-resistant pathogens in the ICU? Ann Intensive Care 2016;6:96. [Crossref] [PubMed]
- Martín-Loeches I, Diaz E, Vallés J. Risks for multidrug-resistant pathogens in the ICU. Curr Opin Crit Care 2014;20:516-24. [Crossref] [PubMed]
- Singh N, Rogers P, Atwood CW, et al. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 2000;162:505-11. [Crossref] [PubMed]
- D'Agata EM, Magal P, Olivier D, et al. Modeling antibiotic resistance in hospitals: the impact of minimizing treatment duration. J Theor Biol 2007;249:487-99. [Crossref] [PubMed]
- Fagon JY, Chastre J, Wolff M, et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med 2000;132:621-30. [Crossref] [PubMed]
- Meersseman W, Lagrou K, Spriet I, et al. Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med 2009;35:1526-31. [Crossref] [PubMed]
- Terraneo S, Ferrer M, Martín-Loeches I, et al. Impact of Candida spp. isolation in the respiratory tract in patients with intensive care unit-acquired pneumonia. Clin Microbiol Infect 2016;22:94.e1-8. [Crossref] [PubMed]
- Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care 2011;15:R267. [Crossref] [PubMed]