Duct ectasia in an accessory breast successfully treated with a flap technique: a case report
Introduction
Duct ectasia is an unusual breast disorder that was described by Zuska in 1951 (1). The condition is also called periductal mastitis, and the pathogenesis is still not clear. One possible mechanism (2,3) is that cellular debris and lipoid material make the terminal ducts beneath the nipple and areola dilated and then extends peripherally. The commonest clinical presentation is nipple discharge and skin retraction with a palpable mass (2,4). As the disease progresses with more ducts and peripheral tissues, inflammatory changes such as mammary duct fistula and sub-areolar abscess may occur in some sever cases (2,5).
The common approach towards duct ectasia is usually conservative. But for some severe cases with duct fistula and extend inflammation which conservative therapy fail, surgery can be applied. Standard surgical excision includes removal of the pathological ducts and the surrounding inflamed tissues (2,5). Incomplete excision always leads to recurrence, whereas complete excision occasionally leads to tissue defects and cosmetic problems. In certain rare severe cases, patients have to undergo mastectomy when several previous excisions fail (6,7).
Herein, we report the first case of duct ectasia in an accessory breast in China. We also introduce a successful flap technique to resolve the dilemma of complete excision and adequate healing in the severe condition after conservative therapy fails. The study was approved by the Institutional Review Board at Peking Union Medical College Hospital in accordance with the latest version of the Declaration of Helsinki and informed consent was obtained from the patient for publication of this case report and any accompanying images.
Case presentation
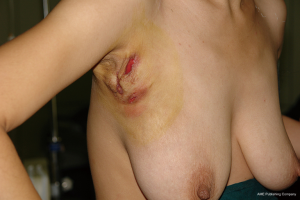
A healthy 39-year-old, non-lactating Chinese female presented with a 1-year history of a recurrent lesion in the right axillary accessory breast (Figure 1). She had received antibiotic therapy and undergone a drainage procedure during the past year that was not successful. She had no history of any inflammation or injuries to her breast and no family history of cancer. Physical examination revealed bilateral axillary accessory breasts and an extensive inflammatory lesion and fistula formation in the right accessory breast. Ultrasound showed an extensive multifocal inflammatory lesion with a size of 10 cm × 7 cm in that breast.
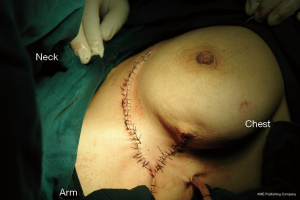
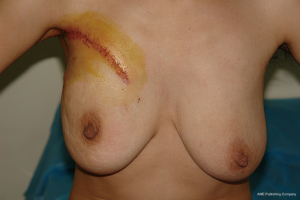
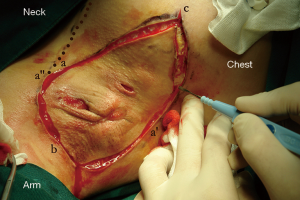
Because of the patient’s severe lesion, we decided on an accessory breast excision procedure. Surgical debridement was performed to remove the entire lesion including the involved skin, fistula, pathologic ducts, inflammatory debris, and necrotic tissues. All affected ducts were completely excised to prevent possible recurrence. However, the challenges of an axillary defect and healing problems presenting after excision remained. A random dermoglandular flap including the full-thickness was outlined and harvested by an additional incision from the upper area with relatively more tissue. Then the mobile end of the flap (point a) was retracted toward the middle of inferior contralateral margin (point a’) of the defect. At last the incision was closed by suturing ba’’ to ba’ and ca to ca’ (Figure 2). Primary suture was achieved (Figure 3). Pathology reported “duct ectasia” after the operation (pathologic specimen revealed the disappearance of lining cells and periductal inflammation predominantly characterized by plasma cells). One month post-operation, the patient exhibited a good healing and good shape (Figure 4). There is no side effect on the activities of the patient’s right shoulder and right arm.
Discussion
The exact pathogenesis of mammary duct ectasia (MDE) remains unclear (8). Ductal obstruction, hyperprolactinemia, smoking, autoimmune disease, infection and trauma have been implicated by certain authors (9-12). Periductal mastitis, comedo mastitis, secretory disease of the breast and plasma cell mastitis are other conditions that have been associated with MDE (2). Several authors have suggested that MDE and periductal mastitis are different stages of one disease process in which periductal mastitis precedes MDE (1,2). However, certain authors have noted that these conditions are two separate entities affecting different age groups and with different etiologies (13). MDE is usually managed conservatively, and surgical treatment can be a choice in some severe cases with duct fistula and extend inflammation (2,5).
The diagnosis of MDE is usually clinical. MDE can manifest in various ways, such as nipple discharge, a deep breast mass, a sub-areolar abscess or a mammary duct fistula, that often respond to conservative therapy but recur later (2,3). The appearance of MDE during ultrasound depends on the stage of disease. MDE can be observed as dilated ducts, a mass, cheesy texture, inflammatory lesions and even an abscess (14). Pathologic findings are characteristically periductal inflammation and duct dilatation. The basal membrane starts to demonstrate small openings, and the adjacent parenchyma reveals an intense inflammatory reaction in which plasma cells may be found in certain cases (15).
The acute process may be temporarily treated with antibiotics or a simple incision and drainage if an abscess has formed. Because the underlying cause is not removed, standard excision is necessary when conservative therapy fails (2,4,5). We and others recommend that excision include the removal of pathological ducts, abscess, surrounding inflamed tissue, fistulae and terminal lactiferous ducts (16,17).
In the present case, the size of the lesion and the axillary location made the case challenging. Healing by secondary intention is associated with a prolonged recovery time, a long wait time and scarring. The described approach with flap coverage represents a novel solution to this challenging clinical problem. The flap covers the defect, helps to optimize healing, prevents recurrence and releases scar tension. Other future applications for this flap include using it to facilitate the surgical treatment of patients with MDE who have a large defect in the breast after inflamed tissue excision.
In sum, we report the first case of MDE in an accessory breast in China and this is the first time which the flap is used in this condition. Our flap technique achieved the complete excision of the extensive inflamed area, primary healing of the large defect and a satisfactory shape. There was no recurrence at the 1-year follow-up.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Zuska JJ, Crile G Jr, Ayres WW. Fistulas of lactifierous ducts. Am J Surg 1951;81:312-7. [Crossref] [PubMed]
- Dixon JM. Periductal mastitis/duct ectasia. World J Surg 1989;13:715-20. [Crossref] [PubMed]
- Haagensen CD. Mammary-duct ectasia; a disease that may simulate carcinoma. Cancer 1951;4:749-61. [Crossref] [PubMed]
- Kamal RM, Hamed ST, Salem DS. Classification of inflammatory breast disorders and step by step diagnosis. Breast J 2009;15:367-80. [Crossref] [PubMed]
- Webb AJ. Mammary duct ectasia--periductal mastitis complex. Br J Surg 1995;82:1300-2. [Crossref] [PubMed]
- Thomas WG, Williamson RC, Davies JD, et al. The clinical syndrome of mammary duct ectasia. Br J Surg 1982;69:423-5. [Crossref] [PubMed]
- Browning J, Bigrigg A, Taylor I. Symptomatic and incidental mammary duct ectasia. J R Soc Med 1986;79:715-6. [PubMed]
- Rahal RM, de Freitas-Júnior R, Carlos da Cunha L, et al. Mammary duct ectasia: an overview. Breast J 2011;17:694-5. [Crossref] [PubMed]
- Peters F, Schuth W. Hyperprolactinemia and nonpuerperal mastitis (duct ectasia). JAMA 1989;261:1618-20. [Crossref] [PubMed]
- Rahal RM, de Freitas-Júnior R, Paulinelli RR. Risk factors for duct ectasia. Breast J 2005;11:262-5. [Crossref] [PubMed]
- Thomas JA, Webster DJ, Williamson ME. Smoking and benign breast cancer. Smoking linked to duct ectasia .. BMJ 1993;307:1146. [Crossref] [PubMed]
- Al-Masad JK. Mammary duct ectasia and periductal mastitis in males. Saudi Med J 2001;22:1030-3. [PubMed]
- Dixon JM, Ravisekar O, Chetty U, et al. Periductal mastitis and duct ectasia: different conditions with different aetiologies. Br J Surg 1996;83:820-2. [Crossref] [PubMed]
- Duchesne N, Skolnik S, Bilmer S. Ultrasound appearance of chronic mammary duct ectasia. Can Assoc Radiol J 2005;56:297-300. [PubMed]
- Tavassoli FA. Pathology of the Breast. 2nd ed. Stamford: Appleton & Lange, 1999.
- Harris JR, Lippman ME, Morrow M, et al. editors. Diseases of the Breast. 3rd ed. Philadelphia: Lippincott Williams and Wilkins, 2004.
- Dixon JM, Kohlhardt SR, Dillon P. Total duct excision. Breast 1998;7:216-9. [Crossref]