Video-assisted versus conventional thoracotomy pneumonectomy: a comparison of perioperative outcomes and short-term measures of convalescence
Introduction
Since first reported in 1933 (1), pneumonectomy has been one of the means for the treatment of lung disease, especially carcinoma. Open approaches have been the gold-standard therapy and were used extensively, however, delayed convalescence may occur after these procedures (2). A new alternative to conventional open approach is video-assisted thoracic surgery pneumonectomy (VATS-P) (3,4).
Video-assisted thoracic surgery (VATS) was initially described more than 20 years ago and have gained an important position in thoracic operations (5). Thoracoscopy surgery, which used to think suitable for patients with early-stage peripheral mass, is gaining an important position in some more complicated situations. However, VATS pneumonectomy is a different procedure because its safety and clinical effect lie in dispute (6). Few reports have described the use of VATS for pneumonectomy (3,6,7-9).
Besides cosmesis, other theoretical advantages of VATS-P include less postoperative pain, lower complications rates and a faster recovery. Comparison of these variables seldom has to be reported. In this paper, we seek to define potential benefits of VATS-P by reporting our comparable experience between VATS-P and conventional pneumonectomy (CP).
Methods
Approval for the study was obtained, and the need for individual patient consent was achieved by the Institutional Review Board. This was a retrospective, case-control study comparing a single surgeon’s experience with 32 VATS-P (cases) performed between March 2013 and August 2016 and 64 CP performed from June 2010 and March 2013 (controls). For lung masses, pneumonectomy was only performed for lesions not able to radical resect the tumour by simple lobectomy or bronchial sleeve resection operation. All VATS-P patients were aware that mini-invasive approach might convert to conventional surgery if necessary.
From June 2010 and March 2013, 150 conventional pneumonectomy were performed at our institution. From this cohort, we selected 64 patients to serve as a control group. These 64 patients were matched in a 2:1 ratio to index VATS-P cases on age, comorbidity, surgical indication (benign vs. malignant), tumour size and lesion location (central or peripheral and right or left). No consideration or analysis of operative parameters and outcomes was done until this group was definitively selected as the best comparison cohort based on preoperative variables only.
Malignant indications for pneumonectomy include tumour invasion of Inter lobe or pulmonary trunk. And single lobectomy cannot radical resect the tumour. Patients received preoperative neoadjuvant therapy (radiotherapy, chemotherapy or both) were exclusive from this study. Benign indications for pneumonectomy was one side of the lung had a broad range of irreparable lesions, such as stenosis of bronchial tuberculosis, bronchial dilation, pulmonary atelectasis, and non-operative treatments were invalid.
The anaesthetic technique for VATS P and CP was same. The CP groups used posterolateral thoracotomy incision. The pulmonary vessels were closed with stitches and bronchus were closed by linear staplers. The mediastinal lymph node dissection was implemented after pneumonectomy for malignant tumour patients. Three surgical ports were utilized in VATS-P: the thoracoscope was placed in the 7th (ICS) at the midaxillary line; the working port (often 4–5 cm) was placed in the 4th ICS at the anterior axillary line; the assistant’s port was put in the 8th ICS between the posterior axillary and subscapular lines. The procedure to perform a left VATS pneumonectomy was divided into following steps: (I) the left superior and inferior pulmonary veins were free using the electrocoagulation hook and suction tip; (II) the pulmonary vein was transected using the endo stapler introduced through the assistant’s port; (III) the pulmonary artery and the left bronchus were successively fully exposed and transected using the endo stapler through the assistant’s port. The range of lymphadenectomy was identical to CP, which included 4 L, 5, 6, 7, 9 and 10 groups. At the end of the surgery, a balanced drainage tube was inserted into the chest wall in proper depth both in VATS-P and CP (usually 4–5 cm).
Both group patients returned to the ICU after surgery. After recovery from anaesthesia and evaluated in stable condition, patients were moved to a regular room. Metoprolol, digoxin and furosemide were routine administrated to avoid acute pulmonary edema after surgery. The chest tube was clipped and opened intermittently. Indications for extubation including the volume of drainage drop below 100 mL and chest X-ray showing the pleural effusion fade below the pulmonary hilar. PCA pumps which contained sufentanil were routinely used in all patients. Visual analogue pain scale (VAPS) scores (1 is no painless and 10 is the worst imaginable pain) were recorded every 8 hours after surgery and continued three days. Hospital discharge was determined when the patient achieved a stable condition enabling self-maintenance of normal daily activities without needing for further treatment.
Demographic information, estimated blood loss (EBL), operation time (OR time), dissected lymph nodes numbers, VAPS, postoperative chest drainage time, the length of stay (LOS), transfusion requirement and postoperative complications were recorded respectively. Mean values were compared using Student’s t-test and frequency distributions were compared using the chi-square test or Fischer’s exact test. A P value of <0.05 was considered to represent a statistically significant difference. Statistical analysis was performed using SPSS ver. 22.0.
Results
Perioperative outcomes are listed in Table 1. A total of 82 males and 14 females were included in this study. Pneumonectomy was performed for destroyed lung in 9 of 96 cases. 87 patients had a radical pneumonectomy for lung carcinoma with a median tumour size of 3.6 cm (range: 1.2–6.4 cm) for both groups. In the VATS-P cohort, 29 patients were diagnosed with the malignant tumour (22 squamous cell carcinoma and seven adenocarcinomas) with a stage distribution of 20 central and nine peripheral lung tumours. In the CP group, 58 cases were the pathologically malignant tumours (44 squamous cell carcinoma and 14 adenocarcinomas), with the distribution of 40 central and 18 peripheral lung tumours. Surgical margins were negative in all cases.
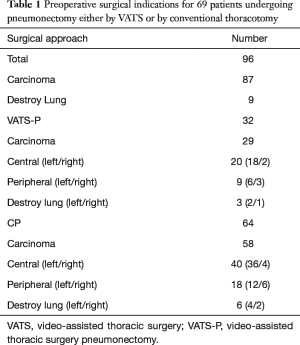
Full table
For malignant tumour patients (Table 2), no differences were found in the ages (55.4 in both group, P=0.99), mean tumour size (3.6 vs. 3.7 cm, P=0.71), postoperative drainage time (5.9 vs. 6.2, P=0.50), LOS (7.5 vs. 8.1, P=0.50), transfusion rates (2% vs. 10%, P=0.50), dissected lymph node numbers (11.9 vs. 14.2, P=0.26), dissected lymph node stations (5.0 vs. 4.9, P=0.75) and mortality rates (0 vs. 0, P=0.10). We did notice a relative low EBL in VATS-P compared with CP (226.0 vs. 261.3, P=0.40). However, the difference showed no significant. VATS-P had lower VAPS (2.1 vs. 2.6, P=0.02) but longer mean operating time (187.5 vs. 146.3 min, P=0.00) than CP. Morbidity of complications between the two groups is almost same (20.0% vs. 22.5%, P=0.82). Cardiovascular and pulmonary complications, particular arrhythmia and pneumonia, were the major postoperative complications in both two group (Table 3). We found no significant difference in the occurrence of each complication. According to the date of operation, all 29 VATS-P patients were divided into three groups (n=9,10,10 respectively). No significant differences were found in the OR time, EBL and Dissected lymph nodes among three groups. We did not found there was a difference in complications changed with the learning curve either (Table 4).

Full table
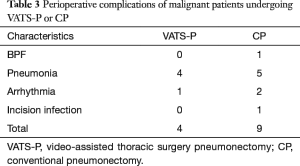
Full table

Full table
For benign lesion (Table 5), all nine patients were combined with destroying lung. Of which, two patients in VATS-P and four patients in CP are tuberculosis lung associated with pulmonary aspergillosis. Over 1,000 mL RBL (1,033.3 vs. 1,233.3, P=0.78) and 180 minutes (186.6 vs. 105.8, P=0.73) OR time were found in both groups. LOS (7.6 vs. 6.3, P=0.57), transfusion rates (66.7% vs. 33.3%), complications rates (zero in both group) and length of drainage (6.7 vs. 6.7, P=1.0) between two groups are almost identical. Lower VAPS (1.7 vs. 2.6, P=0.03) were found in VATS-P comparing to CP.
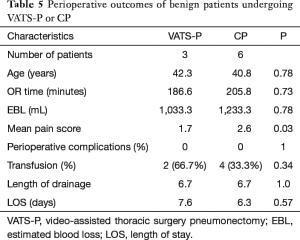
Full table
Discussion
Pneumonectomy brings significant trauma and influence to patients’ quality of life (2). This procedure often followed by postoperative complications with an incidence of 20–60% and many of them were life threatening and require appropriate immediate management (10). For patients with complex inflammatory lung disease, such as tuberculosis, undergoing this procedure was combined with even higher mortality and morbidity (11,12). There has been a recent trend in transforming the convention approach into minimal invasive (13,14) to improve the morbidity and convalescence of pneumonectomy. Previous studies have suggested that VATS lobectomy is an appropriate procedure comparing to open surgery (15,16).
Initial case reports and small non-controlled series have indicated that VATS pneumonectomy is a feasible alternative to conventional open surgery (7,8,17). To better define the actual benefits of this surgical technique, a comparison between patients undergoing VATS-P and those undergoing the CP is necessary to be done. In this study, we performed a case-control comparison of VATS-P and CP carried out by a single surgeon. In controlling for the surgeon, patient age, tumour size and site of surgery, we attempted to minimise the potential for selection bias between surgical modalities.
For patients with malignant tumours, we did not find a shorter postoperative drainage time and hospital stay or a lower intraoperative bleeding and morbidity rates in VATS-P. However, the VATS-P got a lower postoperative pain but a longer operating time compared to the CP. Although the retrospective design is imperfect, this case-matched study suggests that all measurable perioperative outcomes and short-term measures of convalescence are identical with no distinct advantage for VATS pneumonectomy. One reason, we speculate, is that pneumonectomy carries bigger trauma than lobectomy. Even though a mini-invasive technique was done, post operational trauma cannot be minimised. What more, previous surgical comparison was made mostly for lobectomy and patients with early stage but seldom done for middle-advanced stage patients who account for the majority of our research.
We compared the incidence of each postoperative complications and found that several complications between the two groups were not statistically significant. Arrhythmia, which was thought most common complication post operation (18,19), was not common in both groups. One possibility is that metoprolol and digoxin which have anti-arrhythmic effects were routine administrated in all patients. BPF, which was investigated by several previous studies, was related to the operational side, pulmonary function, the stump closure method, neo adjunctive therapy, postoperative mechanical ventilation, and diabetes (20,21). At first, we believe CPs have theoretical advantages in avoiding the formation of BPF comparing to VATS-P because of the length of the bronchial stump, which were reckoned as a risk factor of BPF, may longer in VATS-P. Whereas, no VATS-P patients were developed into BPF during the perioperative period.
Benign lesion in our study, mostly destroyed lung, often associated with severe pleural adhesion caused by chronic pleurisy. What’s more, the involved lung often complicated with inflammatory lung diseases such as bronchiectasis, tuberculosis, necrotizing pneumonia, lung abscesses, fungal infections, lung gangrene, bronchial stricture, congenital malformations, and mycobacterium infections other than tuberculosis (22). These repeated and chronic infections have made hilar and mediastinal lymph nodes calcification. For those reasons, pneumonectomy for destroyed lungs were harder to manipulate and prone to bleeding (23,24). Those patients were assessed individually in our study. Surgical outcomes in two groups, including EBL, postoperative drainage time, LOS, perioperative transfusion and complications rate were almost same. We firstly believe that conventional open approach has its advantage in permitting less operation time and operative bleeding when separating adhesion. Whereas, OR time and EBL in two groups showed no significant difference. We found the VATS approach has the merits of distinct exposure, especially in separating lung tip and costophrenic angle. However, our benign cases were so limited that a larger study would be needed to detect a difference in outcome variables between the two approaches.
Collectively, these data imply that although VATS pneumonectomy is feasible and efficacious, the conventional way cannot be discarded. In our series, we failed to perform pneumonectomy in patients with neoplasm’s diameter greater than 6cm, suggesting that the open approach is complementary approaches for patients with huge masses. Moreover, pleural calcification, which can be met in some extreme situations, is painful manipulate using VATS approach. However, thoracoscopy can show a more explicit structure than the naked eye. As we speculated, VATS-P has its merit in complete lymphadenectomy, but there are no systematic and objective means to evaluated.
This study has various limitations. First, the study is retrospective that biases inherent might exist in the design. Secondly, statistically, differences may not be significant due to a small sample size. The little population in patients with benign lesions cannot well evaluate the potential benefit of VATS-P in destroyed lungs. Thirdly, our results reflect the experience of a high-volume thoracoscopic surgeon. These findings may be less generalisation to surgeons with less experience in minimally invasive surgery. Lastly, long-term follow-up was not included in our study, which we failed to evaluate the effects of VATS-P in survival.
In conclusion, complete VATS pneumonectomy is a minimally invasive technique of which safety and degree of radical resection are similar to those of conventional thoracotomy. A prospective comparison between VATS-P and standard thoracotomy is needed to identify its role more clearly. And postoperative follow-up must be done to define the long-term benefits of VATS pneumonectomy.
Acknowledgements
Funding: This work was supported by The National Natural Science Foundation of China. ‘[Grant numbers No. 81372515, No. 81401901]’ and The Youth Scientific Research Foundation of Xiangya Hospital ‘[Grant number No. 2015Q1, No. 2013Q2]’.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committee of Xiangya Hospital Central South University (No. 201303177).
References
- Fuentes PA. Pneumonectomy: historical perspective and prospective insight. Eur J Cardiothorac Surg 2003;23:439-45. [Crossref] [PubMed]
- Klemperer J, Ginsberg RJ. Morbidity and mortality after pneumonectomy. Chest Surg Clin N Am 1999;9:515-25. vii. [PubMed]
- Walker WS, Carnochan FM, Mattar S. Video-assisted thoracoscopic pneumonectomy. Br J Surg 1994;81:81-2. [Crossref] [PubMed]
- Augustin F, Maier H, Lucciarini P, et al. Extended minimally invasive lung resections: VATS bilobectomy, bronchoplasty, and pneumonectomy. Langenbecks Arch Surg 2016;401:341-8. [Crossref] [PubMed]
- Hartwig MG, D'Amico TA. Thoracoscopic lobectomy: the gold standard for early-stage lung cancer? Ann Thorac Surg 2010;89:S2098-101. [Crossref] [PubMed]
- Nwogu CE, Glinianski M, Demmy TL. Minimally invasive pneumonectomy. Ann Thorac Surg 2006;82:e3-4. [Crossref] [PubMed]
- Conlan AA, Sandor A. Total thoracoscopic pneumonectomy: indications and technical considerations. J Thorac Cardiovasc Surg 2003;126:2083-5. [Crossref] [PubMed]
- Sahai RK, Nwogu CE, Yendamuri S, et al. Is thoracoscopic pneumonectomy safe? Ann Thorac Surg 2009;88:1086-92. [Crossref] [PubMed]
- Nwogu CE, Yendamuri S, Demmy TL. Does thoracoscopic pneumonectomy for lung cancer affect survival? Ann Thorac Surg 2010;89:S2102-6. [Crossref] [PubMed]
- Chandrashekhara SH, Bhalla AS, Sharma R, et al. Imaging in postpneumonectomy complications: a pictorial review. J Cancer Res Ther 2011;7:3-10. [Crossref] [PubMed]
- Conlan AA, Lukanich JM, Shutz J, et al. Elective pneumonectomy for benign lung disease: modern-day mortality and morbidity. J Thorac Cardiovasc Surg 1995;110:1118-24. [Crossref] [PubMed]
- Blyth DF. Pneumonectomy for inflammatory lung disease. Eur J Cardiothorac Surg 2000;18:429-34. [Crossref] [PubMed]
- Nagai S, Imanishi N, Matsuoka T, et al. Video-assisted thoracoscopic pneumonectomy: retrospective outcome analysis of 47 consecutive patients. Ann Thorac Surg 2014;97:1908-13. [Crossref] [PubMed]
- Chen HW, Du M. Video-assisted thoracoscopic pneumonectomy. J Thorac Dis 2015;7:764-6. [PubMed]
- Higuchi M, Yaginuma H, Yonechi A, et al. Long-term outcomes after video-assisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer. J Cardiothorac Surg 2014;9:88. [Crossref] [PubMed]
- Cai YX, Fu XN, Xu QZ, et al. Thoracoscopic lobectomy versus open lobectomy in stage I non-small cell lung cancer: a meta-analysis. PLoS One 2013;8:e82366. [Crossref] [PubMed]
- Liang Z, Chen J, He Z, et al. Video-assisted thoracoscopic pneumonectomy: the anterior approach. J Thorac Dis 2013;5:855-61. [PubMed]
- Shiono S, Abiko M, Sato T. Postoperative complications in elderly patients after lung cancer surgery. Interact Cardiovasc Thorac Surg 2013;16:819-23. [Crossref] [PubMed]
- Harpole DH, Liptay MJ, DeCamp MM Jr, et al. Prospective analysis of pneumonectomy: risk factors for major morbidity and cardiac dysrhythmias. Ann Thorac Surg 1996;61:977-82. [Crossref] [PubMed]
- Deschamps C, Bernard A, Nichols FC 3rd, et al. Empyema and bronchopleural fistula after pneumonectomy: factors affecting incidence. Ann Thorac Surg 2001;72:243-7; discussion 248. [Crossref] [PubMed]
- Wright CD, Wain JC, Mathisen DJ, et al. Postpneumonectomy bronchopleural fistula after sutured bronchial closure: incidence, risk factors, and management. J Thorac Cardiovasc Surg 1996;112:1367-71. [Crossref] [PubMed]
- Kosar A, Orki A, Kiral H, et al. Pneumonectomy in children for destroyed lung: evaluation of 18 cases. Ann Thorac Surg 2010;89:226-31. [Crossref] [PubMed]
- Kendja F, Tanauh Y, Kouamé J, et al. Surgical management of lungs destroyed by tuberculosis. Rev Pneumol Clin 2006;62:171-4. [Crossref] [PubMed]
- Reed CE. Pneumonectomy for chronic infection: fraught with danger? Ann Thorac Surg 1995;59:408-11. [Crossref] [PubMed]


