Recent advances in diagnostic bronchoscopy
Introduction
Since its introduction in the 1960s, flexible bronchoscopy has become the most commonly used invasive technique for diagnosing and treating diseases of the lungs and bronchi (1,2). Basic bronchoscopic techniques such as bronchoalveolar lavage, endobronchial and transbronchial biopsy, and cytology brushing, continue to be cornerstone in the management of our patients with lung ailments. However, it is the multiple advances in the last decade that have taken the role of bronchoscopy to a much higher level both in the diagnostic and therapeutic arena. This article reviews the latest developments in three key areas of diagnostic bronchoscopy that have experienced rapid growth in recent years: endobronchial ultrasound (EBUS), guided-bronchoscopy, and cryobiopsy.
EBUS
Convex-Probe EBUS is a technique that allows real-time sonographic visualization of peri-bronchial structures during bronchoscopy using an ultrasound transducer which is mounted at the tip of the bronchoscope (3). Its advent at the beginning of the 21st century dramatically changed the world of bronchoscopy. EBUS-guided transbronchial needle aspiration (EBUS-TBNA) has rapidly become the cornerstone for the staging of lung cancer and for the diagnosis of mediastinal and hilar lymphadenopathies. The first clinical trial on EBUS-TBNA was published approximately a decade ago, and since then, the body of literature has grown exponentially. We have previously described two phases of research on EBUS-TBNA that occurred over the last decade (4). In the initial phase [2005–2009], the scientific community evaluated the effectiveness of EBUS-TBNA for diagnosis of mediastinal and hilar lymphadenopathies and for lung cancer staging. Once EBUS-TBNA’s effectiveness was proven and widely accepted, a second phase followed [2010–2014], in which scientists focused on technical aspects that could potentially influence the yield of EBUS-TBNA as well as on different educational strategies. This rapidly growing body of literature has, in fact, led to the publication of two sets of EBUS clinical guidelines (3,5). Clinical trials with head to head comparisons of EBUS-TBNA vs. cervical mediastinoscopy (CM) for mediastinal staging of lung cancer have shown comparable accuracy and, not surprisingly, a more favorable safety profile in favor of EBUS-TBNA (6-8). Moreover, a more recent prospective comparison performed by Um and coworkers showed EBUS to be superior to CM with a sensitivity of 88% vs. 81% and negative predictive value of 85% vs. 79% (P<0.05) (7). The cost effectiveness of EBUS-TBNA over mediastinoscopy has also been shown in several studies (9,10). The mean cost of EBUS-TBNA strategy was $2,998 per patient, whereas the strategy of CM alone was significantly more costly at $5,115 per patient (P<0.001) (9). EBUS-TBNA prevented 97% of mediastinoscopies (95% CI, 83–95%) (9).
Supported by the evidence above, in 2013 the American College of Chest Physicians (CHEST) published the 3rd evidence based clinical practice guidelines (11) in which they recommend a “needle technique” [EBUS-TBNA, endoscopic ultrasound needle aspiration (EUS-NA), or a combined EBUS/EUS-NA] over surgical staging as the first test for invasive mediastinal staging. These recommendations were also adopted by ESTS (European Society of Thoracic Surgery) (12) and NCCN (National Comprehensive Cancer Network) guidelines (13).
Over the past few years, several studies have explored the role of EBUS-TBNA in the diagnosis of lymphomas. Unfortunately the majority of these were performed retrospectively, and their results are inconsistent (14-16). What can be concluded from the available literature is that the sensitivity of EBUS to diagnose “de novo” lymphomas (67–88%) (14,16) is smaller than the sensitivity to diagnose cases of relapse (81–100%) (14,16). Prospective studies are needed to more thoroughly examine the role of EBUS-TBNA in this setting.
The use of EBUS-TBNA in the diagnosis of sarcoidosis has been explored as well. EBUS-TBNA has been found to be the most effective method to diagnose stages I–II of sarcoidosis (17-19). Tremblay and coworkers showed a superior diagnostic yield of EBUS-TBNA in comparison with standard TBNA (18), and the GRANULOMA trial showed EBUS-TBNA to be superior to either endobronchial and transbronchial lung biopsies (19). A study by Gupta and coworkers showed that although transbronchial lung biopsies or endobronchial biopsies can have an additive effect to the diagnostic yield of EBUS-TBNA, EBUS-TBNA remains the single most effective technique (17). Hence, considering that it also has a more favorable safety profile (in comparison with transbronchial biopsies), EBUS-TBNA is likely the initial method of choice to diagnose stages I and II sarcoidosis. Transbronchial and endobronchial biopsies are now typically reserved for patients with a high suspicion for sarcoidosis whose lymph nodes are sampled by EBUS-TBNA and do not show evidence of granulomas.
Another application of EBUS-TBNA that has more recently been studied is the diagnosis of tuberculosis. Tuberculous lymphadenitis is the most common extrapulmonary manifestation of tuberculosis among all ethnic groups in the USA and the UK (20), and can present special challenges especially when conventional diagnostic approaches are negative. Other diseases such as lymphomas and sarcoidosis can be in the differential. Most of the studies available exploring EBUS-TBNA in this clinical context are retrospective, but prospective data are available from tuberculosis endemic populations where the utility of this modality can be of significant importance. In India, where tuberculosis is endemic, a prospective study of 102 patients who underwent EBUS-TBNA showed that the diagnostic yield of EBUS-TBNA in tuberculosis was 84.8% using the criteria of positive acid fast bacilli (AFB) smears, necrotizing granuloma in the setting of positive tuberculin skin test, and GeneXpert MTB-RIF test (Cepheid) (21). However, this was only a subgroup analysis and this study was not specifically designed to study the use of this modality in diagnosing tuberculosis. A smaller prospective study in China, another TB endemic population, involving 59 patients was specifically designed to study the use of EBUS-TBNA in patients with negative AFB smears suspected of tuberculosis (22). EBUS-TBNA had a sensitivity of 85% and a diagnostic accuracy rate of 90%, with 46% of patients found to have positive culture (22). Pathologic findings consistent with TB were found in 80% of patients, and in 27% the smear was positive for AFB (22).
The most recent advances in EBUS, however, are focused on ultrasound imaging and involve the ultrasonographic characteristics of the LN, vascular patterns and elastography, and these will be described in the following sections.
Sonographic analysis of lymph nodes
Though retrospective, the largest study on this topic to date was published by Fujiwara and coworkers (23) assessing a total of 1,061 lymph nodes in 487 patients. Round shape (HR 3.1; 95% CI, 1.79–5.36), distinct margin (HR 3.05; 95% CI, 1.61–5.75), heterogeneous echogenicity (HR 1.96; 95% CI, 1.12–3.40), presence of coagulation necrosis sign (HR 5.64; 95% CI, 3.4–9.38) were found to be independently predictive of malignancy. When all four factors were absent, 96% of the lymph nodes were benign. Interestingly, this study did not find that size >10 mm was an independent predictor. By contrast, Memoli et al. (24) also examined ultrasound characteristics of 227 lymph nodes in 100 patients, and found that lymph nodes measuring 10–20 mm (OR 2.8; 95% CI, 1.11–7.52), and >20 mm (OR 34.38; 95% CI, 6.02–196.48) were associated with malignancy. In contrast to the prior study, distinct margins were not associated with malignancy, although the other characteristics (round, hypoechoic) were significantly associated with increased risk of malignancy (24).
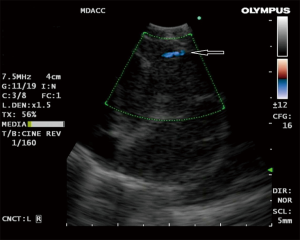
The use of doppler vascular image patterns were examined by Nakajima et al. (25). A classification system was developed based on the pattern and number of vessels in the lymph node graded from 0–III in order of the extent of vessel inflow. When grade 0 (no or minimal flow) and I (few main vessels running towards center of LN) were defined as “benign” and grade II (few punctiform or rod-shaped flow signals) and III (rich flow with more than four vessel with different diameter and a helical flow signal) as “malignant”, they found that the sensitivity and diagnostic accuracy rate were 87.7% and 78% respectively (25). They also described with color-doppler imaging the “inflow sign” consisting of blood arising in bronchial artery and flowing towards the LN (away from the probe) resulting in a blue signal (Figure 1). The accuracy of predicting metastasis solely from a positive BA inflow sign was 80.3%.
EBUS elastography
Recently, a new EBUS processor has been equipped with elastography which allows measurement of tissue compressibility (26). In theory, pathological processes such as malignancy makes tissues less compressible. This modality was first applied to breast ultrasound (27), but has been applied in the thyroid gland (28) and liver cirrhosis (29). The use of elastography in gastrointestinal endoscopy (EUS) has been reported and has been found to have a high sensitivity for detecting neoplasia (30). During elastography, the elasticity of the tissue within the scanned area is compared with the surrounding tissue, and is translated to a color signal that is superimposed on the B-mode image. Colors associated with hard, intermediate and soft tissues are blue, green and yellow/red respectively, with a spectrum displayed in the ultrasound image (26) (Figure 2).
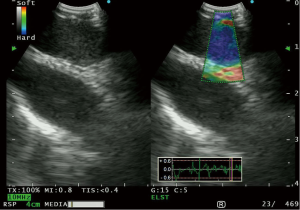
Izumo et al. (31) published the first retrospective data on this modality, and classified lymph nodes into 3 types (Type 1-predominantly non-blue, Type 2-part blue, and Type 3-predominantly blue), in the order of stiffness of the lymph node. They found 94.6% of type 3 lymph nodes to be positive for lymph node metastasis. Rozman et al. (32) also published retrospective data on elastographic analysis of 80 mediastinal lymph nodes. They utilized a “strain ratio” in which the strain measurement of the lymph node was compared to normal appearing soft tissue from the surrounding mediastinum. The area under the ROC curve for the strain ratio was found to be 0.87 (P<0.0001), with a strain ratio of ≥0.8 having a sensitivity of 88.24%, and a diagnostic accuracy of 86.25% for diagnosing malignant lymph nodes. Nakajima and coworkers (33) published prospective data of 41 patients in whom they found a higher stiff area ratio in metastatic compared to benign lymph nodes (P=0.0002). Using a cut off value of a stiff area ratio of 0.31, the sensitivity of elastography was 81%. Interestingly, the stiff area was morphologically compatible with the metastatic distribution in histologically analyzed surgically resected lymph nodes (33).
Elastography is an exciting addition to EBUS, and appears to provide a high sensitivity to distinguish between malignant and benign lymph nodes. However, further studies need to be made to elucidate the incremental benefit if elastography versus conventional B-mode EBUS.
Neither the ultrasound characteristics nor the elastographic appearance are likely to replace the need for biopsy of the LN. Having said so, there are potential scenarios in which this additional information may become valuable. When performing EBUS for mediastinal staging, we often find several LN in a given nodal station and it is not always feasible to sample all of these. These ultrasound/elastographic characteristics may help us determine our best targets. Also, although we typically sample LN that is 5 mm or greater in short axis by EBUS, this cutoff was arbitrarily chosen. We sometimes find LN that do not meet this size criterion (5 mm in short axis) but do have ultrasound/elastographic characteristics of malignancy, and these may need to be sampled.
Guided bronchoscopy
The recent recommendation for lung cancer screening and the increasing use of computed tomography (CT) for thoracic diseases is leading to a rapid rise in the number of lung nodules that are being detected on a yearly basis. Since the majority of these nodules will ultimately be found to be benign, it is crucial to find an accurate and safe approach to establish diagnosis. Currently, transthoracic needle aspiration (TTNA) is the preferred technique due to the diagnostic yield reaching 90%, however, it also carries a pneumothorax rate of approximately 15% of procedures, with the need for chest tube insertion in 5% (34). Standard bronchoscopic technique is safer than TTNA with an overall complication rate of <1% of procedures (35), including pneumothorax. However, it has a lower diagnostic yield, in the range between 18% to 62% (36). This technique is highly dependent on the size of the lesion, its location, and the ability to visualize the lesion with fluoroscopy. Various combinations of bronchoscopic techniques such as radial EBUS, electromagnetic navigation and virtual bronchoscopic navigation have been developed in the past decade to improve the yield of bronchoscopic techniques. We will refer to all collectively as “guided-bronchoscopy” in this article. A recent meta-analysis showed that the pooled diagnostic yield of guided bronchoscopy was 70% (range 45% to 86.2%) across studies (37). In the next few paragraphs we will focus on the newest approaches and most innovative techniques for the bronchoscopic diagnosis of peripheral lung nodules.
Thin and ultrathin bronchoscopy
While the exact diameter range characterizing ultrathin, thin, and conventional bronchoscopes is not clearly defined in the literature, the latter usually have a diameter of 5.0 mm or more. Ultrathin bronchoscopes are typically 3.0 mm or less in outer diameter and were first used in the 1980’s (38-40). Since then, built-in channels were added but allowed for only minimal interventions such as cytobrushing and bronchoalveolar lavage (41,42). Recently, a new generation of thin and ultrathin bronchoscopes were released and are characterized by having larger working channels as well as improved left/right rotation function of up to 120°. The BF-P190 (Olympus, Tokyo, Japan), for example, has a distal end outer diameter of 4.2 mm and an working channel inner diameter of 2.0 mm (Figure 3). While conventional bronchoscopes can visualize up to the 4th generation bronchi, ultrathin bronchoscopes can reach the 6th to 8th generation bronchi and, when combined with radial EBUS, can verify that the peripheral lesion of choice has been reached (37). This combination of enhanced maneuverability, smaller caliber bronchoscopes and larger diameter instrument channels constitutes a major advance in small airway navigation and provides better access to peripheral lung lesions. In a randomized control trial, Oki and colleagues found that the diagnostic yield of transbronchial biopsies performed using multimodal devices (EBUS, fluoroscopy, and virtual bronchoscopic navigation guidance) was higher using the ultrathin bronchoscopy method (74%) compared to the combination of thin bronchoscopy with a guide sheath (59%) (43). While the addition of virtual bronchoscopic navigation alone to ultrathin bronchoscopy did not improve the overall diagnostic yield for peripheral pulmonary lesions, the subgroup of patients with lesions in the right upper lobe or peripheral third of the lung demonstrated higher yield with such a combination (44).

Cone beam computed tomography
Cone beam computed tomography or conebeam-CT (DynaCT, SIEMENS AG Forchheim, Germany) is a novel image modality where image acquisition is achieved by a single, wide beam, X-ray source delivered by a C-arm that rotates 220° as compared to conventional CT imaging that has several, narrow beam, X-ray sources undergoing multiple 360° rotations while the patient is moving horizontally on a table (45,46). As such, the Conebeam-CT is better suited for hybrid interventions, notably because the C-arm provides mobility and maneuverability but also the data collected by Conebeam-CT can be reconstructed and made available immediately to the interventionalists to guide their procedures. Conebeam-CT is the first real-time extra-thoracic navigational modality after conventional fluoroscopy. This technology has already been in use for cerebral aneurysm interventions, cardiovascular therapy, and interventional oncology. It is believed today that it would be able to support the growing field of interventional pulmonology, particularly for the diagnosis of peripheral lung nodules and possibly the treatment of early stage lung cancer. A feasibility study was therefore conducted by Hohenforst-Schmidt and colleagues where they enrolled 33 incidental solitary pulmonary nodules with mean diameter of 25 mm (±12) and shortest distance to visceral pleura of 25 mm (±18). The diagnostic yield of DynaCT navigation-guided transbronchial biopsies was noted to be at least up to twofold higher than conventional transbronchial biopsies for incidental solitary pulmonary nodules that are less than 20 mm in size. For pulmonary nodules that were invisible on conventional fluoroscopy, the diagnostic yield of DynaCT navigation-guided forceps transbronchial biopsies was at least in the range of other navigation studies which were performed partly with multiple navigation tools and multiple instruments (45). While promising at first look, further studies are needed before Conebeam CT is adopted in daily practice.
Bronchoscopic transparenchymal nodule access (BTPNA)
BTPNA is another innovative approach to sampling peripheral lung nodules. This technique allows the bronchoscopist to access the nodule by creating a direct pathway that starts at the airway, goes through lung parenchyma, and directly reaches the lesion. This approach would obviate the need of a bronchi leading to the target (47). In an initial feasibility study conducted in canines, fiducial markers were placed in anesthetized dogs and CT images of the thorax were acquired. Then using the CT scan data, the BTPNA software allowed the construction of an automatic point-of entry with a bronchoscopic tunnel pathway through the lung parenchymal tissue that leads straight to the lesion. The procedure plan was uploaded to a virtual bronchoscopic navigation system that guided the bronchoscopist to the point-of-entry area. There, the airway wall was pierced by an 18-gauge needle and the opening was dilated by a small balloon catheter. Next, a 2.0 mm working channel sheath was inserted into the opening and was locked to a 15-gauge stylet and advanced together to the target lung lesion under fused CT scan-fluoroscopy guidance. The 2.0 mm sheath was then kept in place to allow various instruments to be used to sample the lesion. Thirteen tunnels were created this way in four canines with the average length of the tunnels being 32.3 mm and an average proximity to the target lesion of 5.7 mm. There were no pneumothorax noted and the estimated blood loss was less than 2 mL (47). In a subsequent animal study, the same group assessed the diagnostic yield of BTPNA in a nine-canine cohort that had Radiesse targets (0.25-mL aliquots of calcium hydroxyapatite) implanted into their lung parenchyma. A total of 31 tunnels were created and the diagnostic yield was 90.3% (48). The first human trial was conducted as a feasibility prospective single-arm interventional study. A total of 12 patients were recruited and 10 of them had a tunnel pathway successfully created. The diagnostic yield was 83% with no post-procedural adverse events reported (49). Though this may seem like a promising approach for lesions without “bronchus sign” (airway leading straight to the target), concerns about safety are very valid and further human trials with large sample sizes are needed before adoption of this technique.
Bronchoscopic cryobiopsies
The use of cryoprobes in bronchoscopy were first described in 1977 (50) and have been used for palliative treatment for obstructing endobronchial tumors (51), removal of granulation tissue, inspissated secretions or clot. The cryosurgical equipment relies on the Joule-Thompson effect where compressed gas released at the tip of the cryoprobe rapidly expands and introduces a low temperature.
Recently there has been growing interest in its application to obtain tissue during bronchoscopy. An area where transbronchial cryobiopsy (TBCB) is rapidly gaining interest is in the diagnosis of interstitial lung diseases. Current guidelines (52) recommend multidisciplinary discussion as the diagnostic gold standard for the diagnosis of interstitial lung diseases. When the clinical and radiologic evaluation is non-diagnostic, the greatest impact to the final diagnosis is histopathologic information (53). Surgical lung biopsy has been recommended by the current guidelines as the primary method to obtain histopathologic information (54), and discourages the use of transbronchial biopsy with regular forceps for the diagnosis of interstitial lung diseases due to its overall low yield (37%) (55). However, surgical lung biopsy (either through open thoracotomy or video assisted thoracic surgery), can result in significant morbidity. The 30-day mortality after surgical lung biopsy in patients with interstitial lung disease ranges from 2.7% to 12% (56,57), and in patients with idiopathic pulmonary fibrosis, can be as high as 21.7% (58). Acute exacerbations of ILD have been described after surgical biopsies which can increase substantially its morbidity (59). Due to the high morbidity and mortality of surgical lung biopsies, there is a strong interest in the adoption of an alternative method to provide adequate tissue. Thus, TBCB has garnered interest in filling this role among pulmonologists.
Early reports performed this procedure with patients being endotracheally intubated, but recent studies have advocated the use of rigid bronchoscopy to secure the airway and help manage any potential bleeding. In general, the cryoprobe is introduced into the selected area through a flexible bronchoscope under fluoroscopic guidance (typically within 2 cm of the pleural surface) and the probe is activated to freeze the area for 3 to 6 seconds. The probe and the flexible bronchoscope are then retracted “en-bloc”, the tip of the probe with the sample is then thawed, and the sample is placed in fixative (typically formalin) (50). The number of biopsies obtained varies but ranges between 3 and 6 depending on the report (50). A Fogarty balloon (60), endobronchial blocker or a balloon dilator may be used to tamponade the airway to control the bleeding while the bronchoscope is thawing ex vivo. A chest radiograph is then taken to evaluate for the occurrence of pneumothorax after the procedure.
TBCB has been shown to avoid the crush artifact that occurs in standard transbronchial forceps biopsies and has been shown in multiple studies to provide larger specimens as well (61-65). A recent meta-analysis of 11 studies, encompassing 731 patients, summarized the available data on cryobiopsy in interstitial lung diseases to date (66). Most of the literature available are retrospective, but 8 studies reported diagnostic yield after cryobiopsy tissue was incorporated into a multidisciplinary discussion, with a diagnostic yield of 51–98%, pooled diagnostic yield of 79% (95% CI, 65–93%). The most feared complication of TCB is bleeding, with the frequency of moderate/severe bleeding ranging from 0–78%, pooled frequency 39% (95% CI, 3–76%) (66). Pajares and coworkers (65) compared TBCB with standard forceps biopsies in a randomized control trial and showed that there was no significant difference in the occurrence of pneumothorax or moderate bleeding between the two groups. Lastly, TBCB has also been shown to have a lower median time of hospitalization (2.6 vs. 6.1 days, P<0.0001), and has been shown to have a lower mortality due to adverse events (0.3% vs. 2.7%) in comparison with VATS biopsy (67).
As mentioned above, available evidence is heterogeneous and is derived mostly from retrospective literature. The procedural protocols are also varied which may account for the differences in yield and complication rates between studies. Prospective studies comparing TBCB against surgical lung biopsy with regard to diagnostic yield and safety are currently underway (NCT01714518, NCT01972685) and should provide some standardization of technique, and clarify the role of TBCB in the diagnosis of interstitial lung diseases.
Conclusions
The major and exciting advances in diagnostic bronchoscopy during this new century have heightened the role of pulmonologists in the management of thoracic disease. Surgical and invasive procedures are slowly being replaced by different bronchoscopic techniques which are both effective and safe. Despite the superb yield of EBUS-TBNA, there are continuous efforts from the scientific community to increase its performance even further. Although newer techniques are available in the arena of guided-bronchoscopy for peripheral lung lesions, there is still ample room for improvement. Real-time image navigation and target confirmation are key and several new technologies are currently being studied. Transbronchial cryobiopsies can improve the diagnostic yield offered by conventional transbronchial forceps biopsy for the diagnosis of interstitial lung diseases. Prospective trials comparing TBCB against surgical biopsy are currently under way. Until these results are available, TBCB should be reserved for centers with high level of expertise in bronchoscopy that can manage potentially severe bleeding.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Casal has obtained research funding from Spiration, PneumRx, and Siemens. He is also a consultant for Olympus America. The other authors have no conflicts of interest to declare.
References
- Colt HG, Prakash UB, Offord KP. Bronchoscopy in North America: Survey by the American Association for Bronchology, 1999. J Bronchology Interv Pulmonol 2000.7.
- Prakash UB, Offord KP, Stubbs SE. Bronchoscopy in North America: the ACCP survey. Chest 1991;100:1668-75. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Casal RF, Lazarus DR, Eapen GA, et al. EBUS-TBNA: A Decade of Progress. J Clin Trials 2016;6:264. [Crossref]
- van der Heijden EH, Casal RF, Trisolini R, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014;88:500-17. [PubMed]
- Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577-82. [Crossref] [PubMed]
- Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015;10:331-7. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Navani N, Lawrence DR, Kolvekar S, et al. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med 2012;186:255-60. [Crossref] [PubMed]
- Sharples LD, Jackson C, Wheaton E, et al. Clinical effectiveness and cost-effectiveness of endobronchial and endoscopic ultrasound relative to surgical staging in potentially resectable lung cancer: results from the ASTER randomised controlled trial. Health Technol Assess 2012;16:1-75. iii-iv. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Network NCC. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer (Version 4.2016). Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Grosu HB, Iliesiu M, Caraway NP, et al. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for the Diagnosis and Subtyping of Lymphoma. Ann Am Thorac Soc 2015;12:1336-44. [Crossref] [PubMed]
- Kennedy MP, Jimenez CA, Bruzzi JF, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax 2008;63:360-5. [Crossref] [PubMed]
- Moonim MT, Breen R, Fields PA, et al. Diagnosis and subtyping of de novo and relapsed mediastinal lymphomas by endobronchial ultrasound needle aspiration. Am J Respir Crit Care Med 2013;188:1216-23. [Crossref] [PubMed]
- Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest 2014;146:547-56. [Crossref] [PubMed]
- Tremblay A, Stather DR, Maceachern P, et al. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009;136:340-6. [Crossref] [PubMed]
- von Bartheld MB, Dekkers OM, Szlubowski A, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA 2013;309:2457-64. [Crossref] [PubMed]
- Navani N, Molyneaux PL, Breen RA, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: a multicentre study. Thorax 2011;66:889-93. [Crossref] [PubMed]
- Madan K, Mohan A, Ayub II, et al. Initial experience with endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) from a tuberculosis endemic population. J Bronchology Interv Pulmonol 2014;21:208-14. [Crossref] [PubMed]
- Sun J, Teng J, Yang H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in diagnosing intrathoracic tuberculosis. Ann Thorac Surg 2013;96:2021-7. [Crossref] [PubMed]
- Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest 2010;138:641-7. [Crossref] [PubMed]
- Memoli JS, El-Bayoumi E, Pastis NJ, et al. Using endobronchial ultrasound features to predict lymph node metastasis in patients with lung cancer. Chest 2011;140:1550-6. [Crossref] [PubMed]
- Nakajima T, Anayama T, Shingyoji M, et al. Vascular image patterns of lymph nodes for the prediction of metastatic disease during EBUS-TBNA for mediastinal staging of lung cancer. J Thorac Oncol 2012;7:1009-14. [Crossref] [PubMed]
- Trosini-Désert V, F, Taillade L, et al. Bronchial endoscopic ultrasound elastography: preliminary feasibility data. Eur Respir J 2013;41:477-9. [Crossref] [PubMed]
- Moon WK, Chang RF, Chen CJ, et al. Solid breast masses: classification with computer-aided analysis of continuous US images obtained with probe compression. Radiology 2005;236:458-64. [Crossref] [PubMed]
- Lyshchik A, Higashi T, Asato R, et al. Thyroid gland tumor diagnosis at US elastography. Radiology 2005;237:202-11. [Crossref] [PubMed]
- Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006;55:403-8. [Crossref] [PubMed]
- Janssen J, Schlorer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc 2007;65:971-8. [Crossref] [PubMed]
- Izumo T, Sasada S, Chavez C, et al. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol 2014;44:956-62. [Crossref] [PubMed]
- Rozman A, Malovrh MM, Adamic K, et al. Endobronchial ultrasound elastography strain ratio for mediastinal lymph node diagnosis. Radiol Oncol 2015;49:334-40. [Crossref] [PubMed]
- Nakajima T, Inage T, Sata Y, et al. Elastography for Predicting and Localizing Nodal Metastases during Endobronchial Ultrasound. Respiration 2015;90:499-506. [Crossref] [PubMed]
- Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:108S-130S.
- Zavala DC. Diagnostic fiberoptic bronchoscopy: Techniques and results of biopsy in 600 patients. Chest 1975;68:12-9. [Crossref] [PubMed]
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049-54. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Yamamoto S, Ueno K, Imamura F, et al. Usefulness of ultrathin bronchoscopy in diagnosis of lung cancer. Lung Cancer 2004;46:43-8. [Crossref] [PubMed]
- Tanaka M, Satoh M, Kawanami O, et al. A new bronchofiberscope for the study of diseases of very peripheral airways. Chest 1984;85:590-4. [Crossref] [PubMed]
- Tanaka M. Advances and usefulness of ultra-thin bronchofiberscopes. Keio J Med 1996;45:296-300. [Crossref] [PubMed]
- Kikawada M, Ichinose Y, Minemura K, et al. A study of peripheral airway findings using an ultrathin bronchofiberscope and bronchoalveolar lavage fluid with diffuse panbronchiolitis. Respiration 1998;65:433-40. [Crossref] [PubMed]
- Hasegawa S, Hitomi S, Murakawa M, et al. Development of an ultrathin fiberscope with a built-in channel for bronchoscopy in infants. Chest 1996;110:1543-6. [Crossref] [PubMed]
- Oki M, Saka H, Ando M, et al. Ultrathin Bronchoscopy with Multimodal Devices for Peripheral Pulmonary Lesions. A Randomized Trial. Am J Respir Crit Care Med 2015;192:468-76. [Crossref] [PubMed]
- Asano F, Shinagawa N, Ishida T, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med 2013;188:327-33. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Vogl T, et al. Cone Beam Computertomography (CBCT) in Interventional Chest Medicine - High Feasibility for Endobronchial Realtime Navigation. J Cancer 2014;5:231-41. [Crossref] [PubMed]
- Abi-Jaoudeh N, Fisher T, Jacobus J, et al. Prospective Randomized Trial for Image-Guided Biopsy Using Cone-Beam CT Navigation Compared with Conventional CT. J Vasc Interv Radiol 2016;27:1342-9. [Crossref] [PubMed]
- Silvestri GA, Herth FJ, Keast T, et al. Feasibility and safety of bronchoscopic transparenchymal nodule access in canines: a new real-time image-guided approach to lung lesions. Chest 2014;145:833-8. [Crossref] [PubMed]
- Sterman DH, Keast T, Rai L, et al. High yield of bronchoscopic transparenchymal nodule access real-time image-guided sampling in a novel model of small pulmonary nodules in canines. Chest 2015;147:700-7. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Sterman D, et al. Bronchoscopic transparenchymal nodule access (BTPNA): first in human trial of a novel procedure for sampling solitary pulmonary nodules. Thorax 2015;70:326-32. [Crossref] [PubMed]
- Raparia K, Aisner DL, Allen TC, et al. Transbronchial Lung Cryobiopsy for Interstitial Lung Disease Diagnosis: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Schumann C, Hetzel M, Babiak AJ, et al. Endobronchial tumor debulking with a flexible cryoprobe for immediate treatment of malignant stenosis. J Thorac Cardiovasc Surg 2010;139:997-1000. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Flaherty KR, King TE Jr, Raghu G, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med 2004;170:904-10. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Wall CP, Gaensler EA, Carrington CB, et al. Comparison of transbronchial and open biopsies in chronic infiltrative lung diseases. Am Rev Respir Dis 1981;123:280-5. [PubMed]
- Lettieri CJ, Veerappan GR, Helman DL, et al. Outcomes and safety of surgical lung biopsy for interstitial lung disease. Chest 2005;127:1600-5. [Crossref] [PubMed]
- Tiitto L, Heiskanen U, Bloigu R, et al. Thoracoscopic lung biopsy is a safe procedure in diagnosing usual interstitial pneumonia. Chest 2005;128:2375-80. [Crossref] [PubMed]
- Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J 2001;17:175-9. [Crossref] [PubMed]
- Kondoh Y, Taniguchi H, Kitaichi M, et al. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med 2006;100:1753-9. [Crossref] [PubMed]
- Pajares Ruiz V, Torrego Fernandez A, Puzo Ardanuy C, et al. Use of an occlusion balloon in transbronchial lung cryobiopsy. Arch Bronconeumol 2014;50:309-10. [PubMed]
- Casoni GL, Tomassetti S, Cavazza A, et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One 2014;9:e86716. [Crossref] [PubMed]
- Fruchter O, Fridel L, Rosengarten D, et al. Transbronchial cryobiopsy in immunocompromised patients with pulmonary infiltrates: a pilot study. Lung 2013;191:619-24. [Crossref] [PubMed]
- Griff S, Schonfeld N, Ammenwerth W, et al. Diagnostic yield of transbronchial cryobiopsy in non-neoplastic lung disease: a retrospective case series. BMC Pulm Med 2014;14:171. [Crossref] [PubMed]
- Hagmeyer L, Theegarten D, Treml M, et al. Validation of transbronchial cryobiopsy in interstitial lung disease - interim analysis of a prospective trial and critical review of the literature. Sarcoidosis Vasc Diffuse Lung Dis 2016;33:2-9. [PubMed]
- Pajares V, Puzo C, Castillo D, et al. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: a randomized trial. Respirology 2014;19:900-6. [Crossref] [PubMed]
- Johannson KA, Marcoux VS, Ronksley PE, et al. Diagnostic Yield and Complications of Transbronchial Lung Cryobiopsy for Interstitial Lung Disease. A Systematic Review and Metaanalysis. Ann Am Thorac Soc 2016;13:1828-38. [PubMed]
- Ravaglia C, Bonifazi M, Wells AU, et al. Safety and Diagnostic Yield of Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Diseases: A Comparative Study versus Video-Assisted Thoracoscopic Lung Biopsy and a Systematic Review of the Literature. Respiration 2016;91:215-27. [Crossref] [PubMed]