Multiple cavitating pulmonary nodules: rare manifestation of benign metastatic leiomyoma
Case presentation
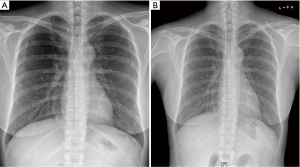
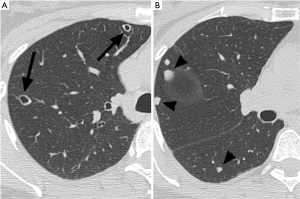
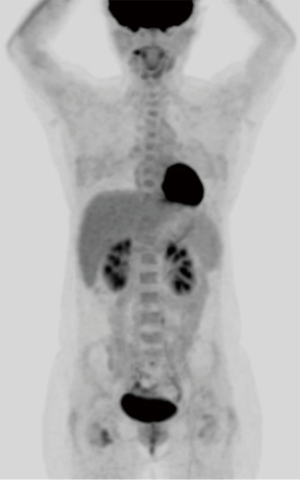
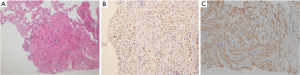
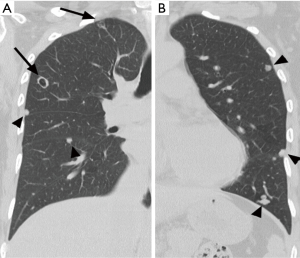
A 52-year-old premenopausal woman presented with multiple bilateral lung cavitations and nodules on a chest X-ray performed for health screening. The patient was a non-smoker, and her general condition was good. The patient had undergone laparoscopically-assisted vaginal hysterectomy 10 years prior to this presentation for leiomyoma of the uterus. The myoma was multiple, intramural type with pathologically proven benign leiomyoma. She had not previously suffered from any pulmonary disease, such as pulmonary tuberculosis or pneumonia. The patient did not exhibit any of the symptoms of pulmonary disease, such as coughing, dyspnea, or chest pain. Her breath sound was clear, and no rales were noted. Because her chest X-ray was abnormal, a chest computed tomography (CT) was performed. Her CT identified multiple cavitary lesions of 5–12 mm in diameter and well-defined nodules of 5–10 mm in diameter in both lung fields (Figure 1). Abdominal-pelvic CT, esophago-gastro-duodenoscopy (EGD), and positron emission tomography (PET)-CT were performed to determine whether the lesions were primary or metastatic neoplasms; however, there were no signs that the lesions were malignant (Figures 2,3). The results of the oxygen saturation test and blood chemistry tests, including carcinoembryonic antigen (CEA) and carbohydrate antigen 125 (CA-125) measurements, were all within the normal limits. However, the patient’s neuron-specific enolase (NSE) level was slightly increased to 24.4 ng/mL (normal range, 0–16.4 ng/mL). Transthoracic needle biopsy was performed on a 10 mm nodule located in the right lower lobe. The pathologic examination revealed a spindle cell bundle, and the mass was positive for estrogen receptor (ER) and progesterone receptor (PR) by immunohistochemical (IHC) staining. The mass was positive for actin and desmin suggesting leiomyoma (Figure 4). Neither mitosis nor atypia were noted, and the immunohistochemistry results for markers of pulmonary carcinoma, including thyroid transcription factor-1 (TTF-1) and pan-cytokeratin, were also negative. Based on the physical examination, imaging studies and pathologic diagnosis of the lung nodule, the final diagnosis was benign metastasizing leiomyoma (BML). The patient underwent subcutaneous injection of a gonadotrophin releasing hormone (GnRH) agonist for 12 months, and her bone mineral density was monitored from 6 months and throughout the treatment. Follow-up low-dose chest CT scan at 15 months revealed decreased cavitations and nodular lesions (Figure 5). The patient tolerated the treatment and had no symptoms throughout 30 months of follow-up. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Discussion
First reported in 1939 by Steiner (1), BML is a rare disease. It is a rare tumor composed of proliferative smooth muscle cells that are pathologically benign, but the tumor is characteristically malignant from the clinical perspective, as it metastasizes to other organs. BML particularly affects women with a history of surgical management on uterine myoma, such as hysterectomy or myomectomy (2-4). In most cases, it affects the lungs (2-8), but other reported sites include the lymph nodes, heart, skull, inferior venae cava, backbone, retroperitoneum and bones (9). The tumor is considered metastatic because it forms multiple remote metastatic lesions, but it is benign pathologically because it has a low mitotic index, lacks diverse nuclei, and does not infiltrate into the surrounding tissues (2). However, the tumor exhibits clinically malignant characteristics, as it originally arises from the uterus and metastasizes to other organs, which accounts for the ironic terminology of BML (2,10,11).
BML is a rare condition and commonly found in women of 35–55 years of age, 3 to 20 years (median: 14.9 years) after the patient underwent hysterectomy or uterine myomectomy (10,12). It typically occurs in reproductive-aged women, but some cases have been reported in pregnant and postmenopausal women. Most cases are asymptomatic and are accompanied by a pelvic mass (13,14), but in some cases, BML is identified because of respiratory or gastro-intestinal symptoms, including chest stiffness, massive hemoptysis or intestinal obstruction (8,15,16). Three hypotheses have been proposed to explain the pathogenesis of BML: the first is that BML is a malignant uterine sarcoma that had gone underdiagnosed pathologically as benign uterine myoma and had subsequently metastasized to the lung. The second is that it is a benign uterine myoma that embolized mechanically during surgical instrumentation to the lung, and the third is that it is a lesion caused by increased growth of smooth muscle cells in various organs, such as the uterus or lungs, due to an abnormally elevated level of estrogen (17). Tietze et al. performed analyses of leiomyoma of the uterus, metastasizing leiomyoma of the lung and disseminated abdominal leiomyomatosis and commonly found an increased number of smooth muscle cells originating from a single cell and inactivation of the same X chromosome in those cells; this generates a higher probability of metastatic pathogenesis than multiple pathogenesis for BML (13). At present, benign pulmonary metastasizing leiomyoma (BPML) is generally classified as a type of leiomyomatosis that mostly involves the uterus and lungs. To obtain a diagnosis of BPML, it is essential to exclude pathologically malignant uterine sarcoma that metastasized to the lung and we should not overlook evidence of sarcoma by re-examining numerous tissue samples of the prior primary leiomyoma of the uterus. In comparison of ten cases of BPML with two cases of uterine sarcoma that metastasized to the lung, the BPML commonly occurred in premenopausal women and rarely invaded both lungs, with lung lesions being found at a median of 14.9 years after hysterectomy (12). In contrast, uterine sarcoma commonly metastasized to the lung in menopausal women and typically invaded both lungs, with lung lesions being found much sooner after hysterectomy than for BPML at a median of 4.5 years after hysterectomy (15). Generally, features that indicate benign lesions rather than malignant lesions include cell death, dysplastic karyotype and various histological morphologies, among which cell division activity is considered the most important (15). When compared to sarcoma, BPML has lower mitotic activity, higher expression rates of ER and PR, and lower angiogenesis (13).
BPML patients display diverse symptoms: often, BPML is revealed incidentally without symptoms, but occasionally mild fever, dry cough, and sputum are observed as early signs. Rarely, patients suffering from BPML come to the clinic as first-time visitors for the evaluation of dyspnea due to heart invasion or respiratory failure due to obstruction of the trachea caused by a large cystic myoma formed in the lung (14-16). The typical radiological finding of BPML is well-circumscribed pulmonary nodules with multiple boundaries; rarely reported radiological findings include a miliary pattern (18), cavitary lung nodules (19,20), interstitial lung disease and multiloculated fluid-containing cystic lesions (6). In summary this atypical presentation of cavitating pulmonary nodules provides interesting diagnostic challenge. Therefore we should take into consideration this rare disease entity of BPML with diverse radiologic findings of lung, multiple cavitations as in this case especially in women who had undergone myomectomy or hysterectomy for uterine leiomyoma.
Acknowledgements
Funding: This study was supported by NRF-2015R1C1A1A02038010.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Steiner PE. Metastasizing fibroleiomyoma of the uterus: Report of a case and review of the literature. Am J Pathol 1939;15:89-110.7.
- Gal AA, Brooks JS, Pietra GG. Leiomyomatous neoplasms of the lung: a clinical, histologic, and immunohistochemical study. Mod Pathol 1989;2:209-16.
- Ma H, Cao J. Benign pulmonary metastasizing leiomyoma of the uterus: A case report. Oncol Lett 2015;9:1347-50.
- Wei WT, Chen PC. Benign metastasizing leiomyoma of the lung: A case report and literature review. Oncol Lett 2015;10:307-12.
- Funakoshi Y, Sawabata N, Takeda S, et al. Pulmonary benign metastasizing leiomyoma from the uterus in a postmenopausal woman: report of a case. Surg Today 2004;34:55-7. [Crossref]
- Maredia R, Snyder BJ, Harvey LA, et al. Benign metastasizing leiomyoma in the lung. Radiographics 1998;18:779-82. [Crossref]
- Esteban JM, Allen WM, Schaerf RH. Benign metastasizing leiomyoma of the uterus: histologic and immunohistochemical characterization of primary and metastatic lesions. Arch Pathol Lab Med 1999;123:960-2.
- Chen S, Liu RM, Li T. Pulmonary benign metastasizing leiomyoma: a case report and literature review. J Thorac Dis 2014;6:E92-8.
- Shida T, Yoshimura M, Chihara H, et al. Intravenous leiomyomatosis of the pelvis with reextension into the heart. Ann Thorac Surg 1986;42:104-6. [Crossref]
- Banner AS, Carrington CB, Emory WB, et al. Efficacy of oophorectomy in lymphangioleiomyomatosis and benign metastasizing leiomyoma. N Engl J Med 1981;305:204-9. [Crossref]
- Hendrickson MR, Kempson RL. Surgical pathology of the uterine corpus. Major Probl Pathol 1979;12:1-580.
- Schneider T, Kugler C, Kayser K, et al. Benign, pulmonary metastatic leiomyoma of the uterus. Chirurg 2001;72:308-11. [Crossref]
- Tietze L, Günther K, Hörbe A, et al. Benign metastasizing leiomyoma: a cytogenetically balanced but clonal disease. Hum Pathol 2000;31:126-8. [Crossref]
- Abramson S, Gilkeson RC, Goldstein JD, et al. Benign metastasizing leiomyoma: clinical, imaging, and pathologic correlation. AJR Am J Roentgenol 2001;176:1409-13. [Crossref]
- Maheux R, Samson Y, Farid NR, et al. Utilization of luteinizing hormone-releasing hormone agonist in pulmonary leiomyomatosis. Fertil Steril 1987;48:315-7.
- Arai T, Yasuda Y, Takaya T, et al. Natural decrease of benign metastasizing leiomyoma. Chest 2000;117:921-2. [Crossref]
- Goyle KK, Moore DF Jr, Garrett C, et al. Benign metastasizing leiomyomatosis: case report and review. Am J Clin Oncol 2003;26:473-6. [Crossref]
- Orejola WC, Vaidya AP, Elmann EM. Benign metastasizing leiomyomatosis of the lungs presenting a miliary pattern. Ann Thorac Surg 2014;98:e113-4. [Crossref]
- Loukeri AA, Pantazopoulos IN, Tringidou R, et al. Benign metastasizing leiomyoma presenting as cavitating lung nodules. Respir Care 2014;59:e94-7. [Crossref]
- Jolissaint JS, Kilbourne SK, LaFortune K, et al. Benign metastasizing leiomyomatosis (BML): A rare cause of cavitary and cystic pulmonary nodules. Respir Med Case Rep 2015;16:122-4.


