Survival after subsequent non-Hodgkin’s lymphoma and non-small cell lung cancer in patients with malignant thymoma
Introduction
Thymomas are rare neoplasms arising from the epithelial cells of the thymus. In the United States, they have an overall incidence of just 0.15 cases per 100,000 person years (1). An increased incidence of subsequent neoplasms after malignant thymoma (MT) is well-described (2-5). This risk appears unrelated to history of thymectomy, radiation therapy, or myasthenia gravis (6-8). Rather, it is thought that the tendency towards subsequent neoplasms is an intrinsic oncological susceptibility that accompanies the increased risk of myasthenia gravis in this patient population (3).
Our group and others have previously used the population-based Surveillance, Epidemiology, and End Results (SEER) program to conduct survival analyses of patients who develop subsequent neoplasms. In those with a history of Hodgkin’s lymphoma, we have found that the history of Hodgkin’s lymphoma adversely impacts survival among patients with subsequent primary breast, non-small cell lung (NSCLC) and head and neck cancers (9-11). A survival analysis of patients registered to the SEER program has also been done with subsequent primary non-Hodgkin’s lymphoma (NHL) after all first primary cancers collectively (12).
To date, there have been a number of population-based studies demonstrating increased risk for subsequent neoplasms after thymoma. However, there have been no studies specifically examining outcomes for specific subsequent neoplasms in this patient population when compared to those patients with the analogous first primary cancer. This has been complicated by the fact that a wide variety of subsequent neoplasms have been reported amongst thymoma survivors (1-8,13-15). These include NHLs, soft tissue sarcomas, gastrointestinal malignancies, prostate cancer, breast cancer, lung cancer, and skin cancer; NHLs and lung cancer are among the most commonly identified.
In the current study, we examined population characteristics among 2,913 survivors of thymoma who later developed NHL and NSCLC, and evaluated the impact of a history of thymoma on survival after NHL and NSCLC by comparing each of these patient cohorts with patients with a first NHL or NSCLC, respectively. We chose NHL and NSCLC because they are established subsequent neoplasms after thymoma. We hypothesized that a history of thymoma would adversely affect overall survival (OS) with both NHL and NSCLC because the increased risk of subsequent neoplasms in thymoma survivors appears to be a function of intrinsic oncological susceptibility and underlying impaired T-cell immunosurveillance, thus raising concern about more aggressive subsequent primary neoplasms. Alternatively, after receiving thymectomy and radiation therapy for thymoma, the curative intent of subsequent malignancies may be limited by the effects of previous treatments.
Methods
Patients
We used the US population-based SEER database [1973–2013] to identify patients who developed NHL or NSCLC after a diagnosis of MT. The SEER database currently covers approximately 30% of the United States population (16). To calculate observed-to-expected ratios (O/E), the SEER program uses 9 registries (SEER-9), which we used to calculate O/E ratios for NHL and lung cancer after MT. For all other analyses, we used the SEER-18 databases. We queried the SEER database for all cases of first primary MT using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) codes for both site and histology. We included those cases with site C37.9 (“thymus”) and histologies 8580/3 (“thymoma, malignant NOS”), 85851/3 (“thymoma, malignant type A”), 8582/3 (“thymoma, malignant type AB”), 8583/3 (“thymoma, malignant type B1”), 8584/3 (“thymoma, malignant type B2”), and 8585/3 (“thymoma, malignant type B3”). We excluded codes indicating thymic carcinoma, namely 8586/3 (“thymic carcinoma, NOS”), 8588/3 (“spindle epithelial tumor with thymus-like element”), and 8589/3 (“carcinoma showing thymus-like element”). Thymic carcinomas were excluded from the analysis due to their distinct histopathology, propensity for capsular invasion and metastases, prognosis, and treatment (17), as has been done in other studies (4,18). While the SEER program specifies that MT is a histopathologic diagnosis independent of invasion (19), published studies have interpreted the codes for malignancy to represent invasion even without true histopathologic malignancy (18,20). The latter assumption is logical as types A and AB generally represent benign histologies (17) and those registering patients to SEER may be apt to score these patients as malignant if the clinical records suggestion invasion.
We defined NHL using the SEER site recode ICD-O-3/WHO 2008: “non-Hodgkin lymphoma”. Of 2,913 patients registered with a first primary MT in the SEER-18 database, 21 patients developed a subsequent primary NHL. For the first primary comparison group, we identified 276,010 patients with a first primary NHL from the same SEER registries, of which 2,697 were excluded due to unknown survival time for a total of 273,313.
We defined NSCLC as occurring at the “site recode ICD-O-3/WHO 2008”: “lung and bronchus”. In addition, the following histological types were included: squamous cell carcinoma (SCCa) (ICD-O-3 codes 8050–8084/3), adenocarcinoma (8140/3, 8255/3, 8260/3, and 8310/3), bronchioloalveolar adenocarcinoma (8250–8254/3), adenosquamous carcinoma (Adenosquamous Ca) (8560/3), large cell carcinoma (8012/3), and non-small cell carcinoma (8046/3). As has been done previously (9), we excluded patients with all other histologies, including neuroendocrine, carcinoid, small cell carcinoma, and unspecified, because the etiologies, natural history, treatment, and/or prognoses differ from those of NSCLC. Of 2,913 patients registered with a first primary MT in the SEER-18 database, 38 patients developed a subsequent primary NSCLC. For the first primary NSCLC comparison group, 569,506 patients with a first primary NSLCLC were identified from the same registries. A total of 2,686 were excluded due to unknown survival time for a total comparison cohort of 566,819.
For both NHL and NSCLC, we required a minimum 2-month latency between thymoma and subsequent primary neoplasm diagnosis, which is the standard latency adopted by SEER to exclude synchronous primary cancers (15). We made no a priori assumptions about the etiology of NHL or NSCLC and so did not otherwise require a minimum latency between the first and subsequent primary cancers.
Statistical analysis
O/E ratios were calculated using the SEER Multiple Primary-Standardized Incidence Ratios tool in SEER*Stat 8.3.2, which accounts for age, sex, race, and year of diagnosis in determining expected rates of the subsequent cancer. Of note, O/E ratios presented for subsequent primary lung malignancy include all lung cancer histologies, including those we excluded from the survivorship analysis. NSCLCs comprise the vast majority of lung cancers.
We assessed associations among tumor and patient characteristics using chi-square tests of independence when expected counts within a cell were greater than five in at least 20% of the cells. For 2×2 contingency tables, the Yates continuity correction was performed. In cases where chi-square testing was not possible, Fisher exact tests were employed (with category binning as necessary). Kaplan-Meier curves were used to describe the OS of various patient groups. The effect of a history of MT on NHL was assessed using Cox proportional hazards modeling, adjusting for radiation, gender, age, extranodal disease, and stage of disease. The effect of a history of MT on NSCLC analysis was adjusted for radiation, gender, age, and stage of disease. All survival analyses were conducted using STATA software (College Station, TX, USA). All P values are two-sided and P<0.05 indicates statistical significance. The University of Rochester Institutional Review Board was not required to approve this study because all data in the SEER database is de-identified and the investigators did not participate in the process of data collection or entry into the SEER database.
Results
O/E ratio for NHL and lung cancer
Higher than expected numbers of observed cases of subsequent primary NHL and lung cancer were found among survivors of MT based on 1,464 patients followed for 10,894 person-years at risk in the SEER-9 database. Thirteen patients developed NHL after MT, whereas 4.95 were expected. The O/E ratio was 2.63 [95% confidence interval (95% CI), 1.40–4.49; P<0.05]. Thirty-six patients developed lung cancer after MT, whereas 18.96 were expected, for an O/E ratio of 1.90 (95% CI, 1.33–3.63; P<0.05).
Patient and tumor characteristics
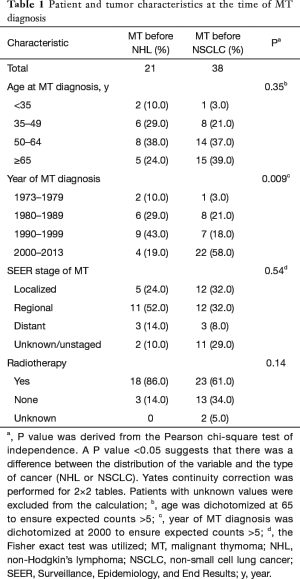
Table 1 shows patient and MT characteristics for patients with subsequent primary NHL after MT (MT-NHL) and subsequent primary NSCLC after MT (MT-NSCLC) at the time of diagnosis of MT. Patients with MT-NSCLC tended to be diagnosed more often in the 2000s relative to those with MT-NHL (P=0.009). There was not an appreciable difference in thymoma stage between the two cohorts. There was a trend towards age of diagnosis greater than 65 in the MT-NSCLC cohort relative to the MT-NHL cohort, and patients with MT-NHL were slightly more likely to have radiation therapy than those with MT-NSCLC, but these findings were not statistically significant. The latency periods between first and subsequent primary cancers are shown in Tables 2 and 3.
Full table
Full table
Full table
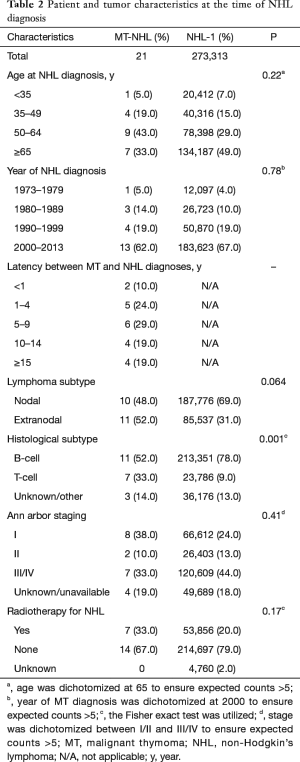
Table 2 summarizes patient and tumor characteristics for NHL in both the MT-NHL and NHL-1 cohorts. Those with MT-NHL presented with T-cell histologies at a higher frequency than those with NHL-1, in which the vast majority were B-cell histologies (P=0.001). The following trends were noted but lacked statistical significance. Patients in the MT-NHL cohort tended to develop NHL at a younger age than those with NHL-1. The MT-NHL cohort tended to have extranodal disease more often than those with NHL-1. Patients with MT-NHL tended to present with less advanced disease by Ann Arbor staging than those with NHL-1. Finally, those with MT-NHL were slightly more likely to receive radiation therapy than those with first primary NHL.
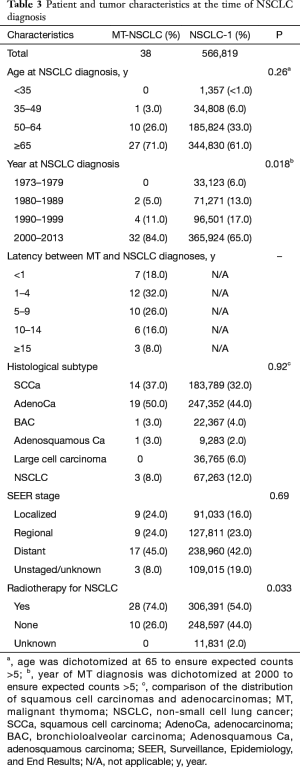
Table 3 summarizes patient and tumor characteristics for NSCLC in both the first NSCLC-1 and MT-NSCLC cohorts. MT-NSCLC patients developed NSCLC in the year 2000 or later more often than those with NSCLC-1 (P=0.018). Patients with MT-NSCLC were more likely to receive radiation therapy than those with NSCLC-1 (P=0.033). There was no appreciable difference in staging between the patients in the two cohorts. The following trends were noted but lacked statistical significance. Patients in the MT-NSCLC cohort tended to develop NSCLC at a more advanced age. Those with MT-NSCLC tended towards adenocarcinoma histology slightly more often than SCCa when compared to the NSCLC-1 cohort.
Impact of a prior history of MT on survival in NHL patients
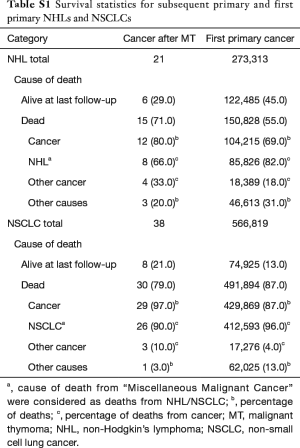
Crude survival statistics are provided (Table S1). Notably, a greater proportion of the patients in the NHL after thymoma cohort died of cancers other than NHL. Of these four patients, two died from cancer of the lung/bronchus, one from Hodgkin lymphoma, and one from acute lymphocytic leukemia.
Full table
The survival curves for the MT-NHL and NHL-1 cohorts and MT-NSCLC and NSCLC-1 cohorts are presented in Figure 1A,B, respectively. Median survival of patients with MT-NHL was 59 months, whereas for patients with NHL-1 it was 90 months. Median survival of patients with MT-NSCLC was 18 months, whereas for patients with NSCLC-1 it was 11 months.

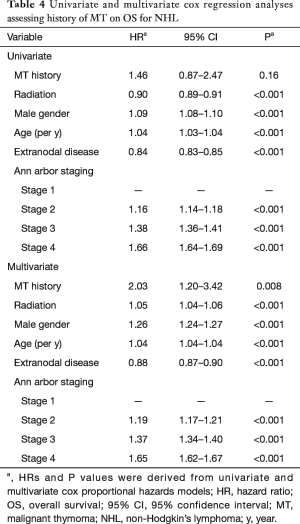
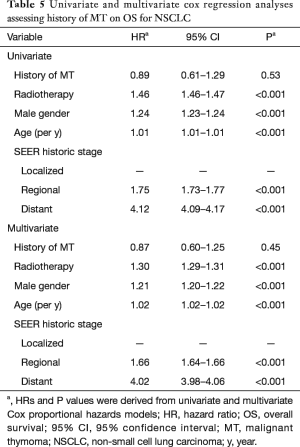
The results of the Cox regression analysis for the NHL and NSCLC cohorts are found in Tables 4 and 5. On univariate analysis, a history of MT was not found to have an adverse prognostic impact on OS for NHL or for NSCLC. The hazard ratio (HR) for OS for a history of MT was 1.46 (95% CI, 0.87–2.47; P=0.16) among patients with NHL and 0.89 (95% CI, 0.61–1.29; P=0.53) among patients with NSCLC. On multivariate analysis, a history of MT was found to be an adverse prognostic factor for OS for NHL but not for NSCLC. The HR for OS for a history of MT was 2.03 (95% CI, 1.20–3.42; P=0.008) among patients with NHL, and 0.87 (95% CI, 0.60–1.25; P=0.45) among patients with NSCLC.
Full table
Full table
Discussion
Increased risk of subsequent primary NHL and lung cancer after MT
We determined an increased risk of both NHL and lung cancer in patients with a history of thymoma relative to what would be expected in the general population. Although the increased risk of subsequent neoplasms after thymoma in general is widely accepted, there is disagreement as to which specific cancers these patients develop at an increased frequency. NHL is one of the most widely reported subsequent neoplasms after thymomas (1,4,5,15), a finding reproduced in the present study. Lung cancer has similarly been widely reported as a subsequent neoplasm after thymoma (3,4,14).
History of MT as putative prognostic factor for NHL
An important finding of the current study is that on multivariate analysis, OS for NHL after thymoma is significantly inferior compared to those patients with first primary NHL. To our knowledge, this is the first study that compares these two groups. Given the relative rarity of thymomas, such a study requires large, population-based registry such as SEER. Pulte et al. also used SEER to describe worse survival for NHL after all first primary cancers, but they did not perform subgroup analyses stratifying by type of previous malignancy (12).
Potential explanations for our findings include different tumor biology in subsequent NHL, increased deaths from other cancers, and less aggressive care with subsequent NHL because of perceived or real excess risk with cumulative therapy. Those patients with NHL after thymoma likely have a different host immune response than those with first primary NHL, potentially giving rise to more aggressive tumor histologies. The thymus, via positive and negative selection, normally gives rise to a population of mature T-cell lymphocytes. Those patients with thymoma have dysfunction of the mechanisms supporting T-cell maturation, and consequently they likely have impaired cancer immunosurveillance (4,20). It is known that other populations with T-cell immunodeficiency such as acquired immunodeficiency syndrome (AIDS) patients and transplant recipients are at increased risk for subsequent NHL (21). Functionally impaired immunosurveillance may also predispose them to more aggressive tumor biology than those patients with de novo NHL. For example, in patients with human immunodeficiency virus-associated NHL, advanced immunodeficiency is a risk factor for worse survival (22). Similar mechanisms may further predispose patients with subsequent NHL after thymoma to third primary cancers, a significant cause of mortality in our MT-NHL cohort.
It is also possible that MT-NHL patients received less aggressive care or even less than the standard of care than those patients with first primary NHL because of concerns with regard to cumulative toxicity. Surgery is the mainstay of treatment for thymomas, with neoadjuvant or adjuvant radiation therapy and chemotherapy given in certain situations (23). There are a host of potential explanations for why thymoma treatment may limit the aggressiveness of treatment for subsequent NHL, including perceived or real concern for excess toxicity due to cumulative toxicity; for example, radiation tolerance for organs-at-risk and surgical or radiation-induced fibrosis. Although our MT-NHL cohort received radiation therapy at comparable rates to the NHL-1 cohort, the treatment plan for radiation therapy is not included in the SEER database.
NSCLC prognosis unaffected by history of MT
Conversely, OS is not significantly different for patients with MT-NSCLC and NSCLC-1. As with subsequent primary NHL, we are not aware of any previous investigations that compare those patients with NSCLC after thymoma and those patients with de novo NSCLC. Our group has used similar methods previously to compare those with NSCLC after Hodgkin’s lymphoma to those with de novo NSCLC (9). In that study, we described a previous history of HL as an adverse prognostic indicator for patients with subsequent primary NSCLC. An important distinction is that radiation therapy is a known risk factor for NSCLC after HL (24), whereas it is not thought to influence the formation of subsequent neoplasms after thymoma (3,7,13). It is possible that the underlying immunological dysfunction in thymoma patients predisposes them to subsequent neoplasms of varying aggressiveness depending on the pathogenesis, which would potentially explain why MT-NHL patients do worse than NHL-1 patients but there is no difference between MT-NSCLC and NSCLC-1 patients. For example, the development of NHL in immunosuppressed patients is related to persistent Epstein-Barr virus infection, whereas lung cancer is not directly linked to infection (25). Future studies will be necessary to clarify the degree of impaired cancer immunosurveillance in thymoma survivors.
Another potential explanation is that oncogenesis after thymoma is multifactorial, with contributions from both intrinsic immunological dysfunction and prior therapy. Previous studies that did not demonstrate a link between radiation therapy and subsequent malignancies in thymoma survivors have generally not looked at subsequent neoplasms individually. It is possible that radiation therapy increases predisposition towards NSCLC but not NHL in thymoma survivors, and that this is related to the radiation field. For example, adjuvant radiation therapy after thymectomy to the post-operative cavity could result in a full dose to a small portion of the adjacent lung and a mid-to-low dose to a larger volume. Hence, it is possible that radiation therapy predisposes to NSCLC after thymoma even if prior studies have not determined radiation therapy to be a risk factor for subsequent malignancies after thymoma. Additional studies will be needed to further clarify late radiation effects of thymoma treatment.
Patient and tumor characteristics
Patients in the MT-NHL cohort tended to present with T-cell histologies more often than those in the NHL-1 cohort, though B-cell histologies comprised the majority in both cohorts individually. In contrast, all 7 of the NHLs following thymoma reported by Engels et al. were B-cell tumors on immunophenotyping and a majority of them were nodal (the majority of our cases were extranodal) (1). Perhaps there is inherent dysfunction in the systems that regulate T-lymphocyte proliferation in patients developing NHL after thymoma that are not limited anatomically to the thymus, thus giving rise to extrathymic T-cell malignancies. In patients with T-cell lymphocytosis associated with thymoma, the peripheral T-cells do not appear to be secondary to mere “spillover” of tumor-related T-cells, providing further support to this theory of systemic immunological dysfunction (26). Patients in the MT-NSCLC cohort were more likely to receive radiation therapy for their NSCLC than patients with first primary NSCLC. It is possible that clinicians opted for more aggressive therapy regimens when faced with patients with subsequent neoplasms. Notably, there was no difference in the rate of radiation therapy for NHL in the MT-NHL cohort relative to the NHL-1 cohort.
Limitations
SEER has known limitations. Patients may move into and out of a SEER geographical location and thus it is possible to miss some cases of subsequent neoplasms. SEER also lacks information on use of chemotherapy, smoking history, and specifics of radiation therapy. In addition, while the SEER program advises to not include benign thymoma (19), the overlap between the definition of malignant behavior and the coding of malignant behavior within the SEER database is likely imperfect and this may have led to inclusions of benign histologies.
Another limitation of the current study is that the subsequent neoplasm cohorts were relatively small. We likely did not have the power to determine differences among patient and tumor characteristics. While we did account for NSCLC and NHL stage in the multivariate analyses, we were unable to separately group and analyze patients by stage as we had done in prior analyses (9,11). That being said, given the relative rarity of thymomas, this type of study is only possible with a large database such as SEER.
In particular, our novel findings for the subsequent NHL after thymoma are limited by the small sample size of the MT-NHL cohort (n=21). Extranodal versus nodal disease was included in the multivariate analysis but no other elements of histology were included. Moreover, the findings of worse OS were found on multivariate analysis but this finding was not reproduced on univariate analysis. Despite these limitations, our analysis supports a putative link between history of MT and worse survival in NHL patients.
Conclusions
There is a significantly increased risk of NHL and lung cancer after thymoma. In this large population-based study, the first of its kind, we provide evidence that patients with subsequent primary NHL after thymoma have worse OS than those patients with first primary NHL. There is no difference in OS between patients with subsequent primary NSCLC after thymoma and first primary NSCLC.
Acknowledgements
We thank Mrs. Laura Finger for editorial assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Given that no identifying patient information was collected for this study, no Institutional Review Board approval was required.
References
- Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546-51. [Crossref] [PubMed]
- Souadjian JV, Silverstein MN, Titus JL. Thymoma and cancer. Cancer 1968;22:1221-5. [Crossref] [PubMed]
- Welsh JS, Wilkins KB, Green R, et al. Association between thymoma and second neoplasms. JAMA 2000;283:1142-3. [Crossref] [PubMed]
- Weksler B, Nason KS, Mackey D, et al. Thymomas and extrathymic cancers. Ann Thorac Surg 2012;93:884-8. [Crossref] [PubMed]
- Gadalla SM, Rajan A, Pfeiffer R, et al. A population-based assessment of mortality and morbidity patterns among patients with thymoma. Int J Cancer 2011;128:2688-94. [Crossref] [PubMed]
- Owe JF, Cvancarova M, Romi F, et al. Extrathymic malignancies in thymoma patients with and without myasthenia gravis. J Neurol Sci 2010;290:66-9. [Crossref] [PubMed]
- Pan CC, Chen PC, Wang LS, et al. Thymoma is associated with an increased risk of second malignancy. Cancer 2001;92:2406-11. [Crossref] [PubMed]
- Evoli A, Minisci C, Di Schino C, et al. Thymoma in patients with MG: characteristics and long-term outcome. Neurology 2002;59:1844-50. [Crossref] [PubMed]
- Milano MT, Li H, Constine LS, et al. Survival after second primary lung cancer: a population-based study of 187 Hodgkin lymphoma patients. Cancer 2011;117:5538-47. [Crossref] [PubMed]
- Chowdhry AK, McHugh C, Fung C, et al. Second primary head and neck cancer after Hodgkin lymphoma: a population-based study of 44,879 survivors of Hodgkin lymphoma. Cancer 2015;121:1436-45. [Crossref] [PubMed]
- Milano MT, Li H, Gail MH, et al. Long-term survival among patients with Hodgkin's lymphoma who developed breast cancer: a population-based study. J Clin Oncol 2010;28:5088-96. [Crossref] [PubMed]
- Pulte D, Gondos A, Brenner H. Long-term survival of patients diagnosed with non-Hodgkin lymphoma after a previous malignancy. Leuk Lymphoma 2009;50:179-86. [Crossref] [PubMed]
- Travis LB, Boice JD Jr, Travis WD. Second primary cancers after thymoma. Int J Cancer 2003;107:868-70. [Crossref] [PubMed]
- Filosso PL, Galassi C, Ruffini E, et al. Thymoma and the increased risk of developing extrathymic malignancies: a multicentre study. Eur J Cardiothorac Surg 2013;44:219-24; discussion 224. [Crossref] [PubMed]
- Curtis RE, Freedman DM, Ron E, et al. New malignancies among cancer survivors: SEER Cancer Registries, 1973-2000. National Cancer Institute, NIH Publ. No. 05-5302. Bethesda, MD, 2006. Available online: https://seer.cancer.gov/archive/publications/mpmono/MPMonograph_complete.pdf
- Surveillance, Epidemiology, and End Results (SEER) Program. Available online: http://www.seer.cancer.gov/
- Eng TY, Fuller CD, Jagirdar J, et al. Thymic carcinoma: state of the art review. Int J Radiat Oncol Biol Phys 2004;59:654-64. [Crossref] [PubMed]
- Kim E, Thomas CR Jr. Conditional survival of malignant thymoma using national population-based surveillance, epidemiology, and end results (SEER) registry (1973-2011). J Thorac Oncol 2015;10:701-7. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program. Available online: http://seer.cancer.gov/seerinquiry/index.php?page=view&id=20110038&type=q
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59-67. [Crossref] [PubMed]
- Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study group., Bohlius J, Schmidlin K, et al. Prognosis of HIV-associated non-Hodgkin lymphoma in patients starting combination antiretroviral therapy. AIDS 2009;23:2029-37. [Crossref] [PubMed]
- Scorsetti M, Leo F, Trama A, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol 2016;99:332-50. [Crossref] [PubMed]
- Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst 2002;94:182-92. [Crossref] [PubMed]
- Engels EA, Pfeiffer RM, Fraumeni JF Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306:1891-901. [Crossref] [PubMed]
- Barton AD. T-cell lymphocytosis associated with lymphocyte-rich thymoma. Cancer 1997;80:1409-17. [Crossref] [PubMed]


