How should this patient with repeated aspiration pneumonia be managed and treated?—a proposal of the Percutaneous ENdoscopIc Gastrostomy and Tracheostomy (PENlIGhT) procedure
Clinical scenario
An 82-year-old man with dysphagia due to cerebrovascular accident (CVA) presented to the emergency department with complaints of cough and fever for 3 days. The clinical history and imaging confirmed aspiration pneumonia presumably due to impaired airway protection. In addition to his acute presentation, the patient appeared malnourished. Further history revealed that the patient had not been able swallow food normally since his CVA event. The pneumonia progressed rapidly to hypoxic respiratory failure requiring endotracheal intubation and mechanical ventilation. A nasogastric feeding tube was placed within 24 hours after intubation and enteral nutrition was initiated. After 10 days treatment, the patient’s fever resolved and he was able to be weaned to minimal ventilator settings. Imaging revealed resolution of his pulmonary infiltrates and blood routine profile had returned normal. Despite these improvements, his underlying dysphagia remained unchanged. How should this patient be managed and treated? Should we simply discharge him, leaving him at high risk of aspiration pneumonia and malnutrition?
Why is this question important?
Dysphagia resulting from central nervous system (CNS) disease is common (1-3). The most common causes of CNS driven dysphagia are ischemic and hemorrhagic CVA (4), intracranial infection, degenerative diseases and autoimmune disorders affecting the CNS. With the aging population, a substantial proportion of elderly patients are at risk for cerebrovascular disorders, which may greatly compromise the autonomic nervous system of the oropharynx (i.e., bulbar function) (5,6). Consequently, the passage of food along the digestive tract is compromised which can lead to recurrent aspiration and subsequent pneumonitis or pneumonia. Management of patients with chronic aspiration can be quite challenging and include nasogastric tube (NGT) feedings, percutaneous gastrostomy tube placement [percutaneous endoscopic gastrostomy (PEG)], jejunal tube placement and medical management. NGT placement has been a longstanding technique aimed at providing enteral nutrition for these patients. However, its adverse effects include nasal wing, chronic sinusitis, gastrooesophageal reflux (GER), and aspiration pneumonia. More recently, clinicians have adopted the use of PEG tube placement in an effort to overcome this problem, however PEG tube placement has not been shown to decrease aspiration events or mortality (7).
Patients with stroke or other CNS disorders may require tracheostomy tube (TT) placement. Data would suggest that approximately 1.3% of patients status-post CVA underwent TT (8). In some cases, patients with post-CVA may require airway protection due to compromised bulbar function, decreased airway protective reflexes, muscle weakness, and a weak cough. As a result, they cannot reliably clear secretions or maintain a patent airway (9,10). In this situation, TT placement allows for transition from mechanical ventilation (MV) to tracheal collar while maintaining airway patency. Placement of a TT also allows for direct access to the airway and improved suctioning from the lower airways (11). In addition, data suggests that patients are more comfortable with TT than with endotracheal intubation (12), allowing for earlier discontinuation of analgesia and sedation, which is helpful in facilitating patients to awaken, be weaned from ventilation, and begin early mobilization regimens (13,14).
The timing of PEG and TT placement remains controversial with evidence existing that PEG placement is often delayed. The time from onset of CVA and decision to insert PEG was 10 days, and the time between decision and PEG insertion was 12 days (4). Such delay may significantly impair nutritional status of stroke patients with dysphagia, as guidelines recommended early initiation of enteral nutritional support for the critically ill (15,16). There is also evidence that early tracheostomy (ET) can improve patient-important outcomes such as mortality, duration of MV and ICU length of stay (14), however this remains controversial (17). Based on this we propose the combined placement of PEG and TT in the same setting as the Percutaneous ENdoscopIc Gastrostomy and Tracheostomy (PENlIGhT) procedure. This procedure is indicated for patients with recurrent aspiration pneumonia due to bulbar dysfunction or coma. The idea to perform PEG and tracheostomy simultaneously is not new (18-20), which has been shown to expedite recovery of these patients (21). In the next section, we systematically review the literature to obtain the state-of-the-art evidence to support our concept.
Who will benefit from the PENlIGhT procedure?
Patients with persistent swallow disturbances for 1 month after the onset of stroke are eligible for insertion of PEG. Concomitantly, the patient should have impaired cough reflex that they cannot reliably clear secretions from the airway. In clinical practice, high gugging swallowing screen (GUSS) grade (22), dysphonia, an abnormal gag reflex, impaired voluntary cough, incomplete oral-labial closure, a high NIHSS score, or cranial nerve palsies should alert the care team to the risk of dysphagia (23-25). However, a preserved gag reflex may not indicate safety with swallowing (26). The clinical scenario described earlier is often encountered in elderly patients after CVA.
We suggest that the criteria in selecting patients for the PENlIGhT procedure are the need for long-term MV and airway protection and swallowing disturbance. In patients with severe traumatic brain injury, Mandaville and colleagues established a model to predict probability of long-term (>6 weeks) swallowing disturbance. The model contains variables age, Initial Ranchos Los Amigos (RLA) score, TT and initial aphonia (27). A mathematical equation was derived for the prediction purpose:
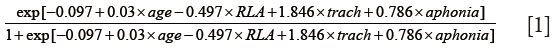
More recently, Faigle and colleagues developed the GRAVo scoring system for prediction of the need for PEG placement in patients with intracranial hemorrhage. The name GRAVo represented the four components of the scoring system: Glasgow coma scale (GCS), Race, Age and Volume. The authors stated that “Points for the GRAVo score were assigned as follows: 2 points for GCS ≤12, 1 point for black race, 2 points for age >50 years, and 1 point for ICH volume >30 mL, with a maximum of 6 points. The PEG placement rates for patients with a score of 0 to 1, 2 to 3, and 4 to 6 were 8.7%, 19.6%, and 63.0%, respectively.” (28). There are many other tools being used for prediction of PEG placement, and components of these tools included age, 24-hour National Institutes of Health Stroke Scale, race and body mass index (29-31).
Similarly, many efforts have been made to predict patients who need tracheostomy (32,33). The stroke-related early tracheostomy score (SET score) was estimated within 24 hours after admission and consisted of neurological function, neurological lesion and general organ function/procedure (11). The TRACH Score was defined by radiological scale (RScale) and Glasgow Outcome Score (GOS) (34). However, there is no tool for the prediction of patients requiring both tracheostomy and PEG, which is an area in need of further investigations.
Evidence for PEG
Search strategy and study selection
Electronic database of PubMed was searched from inception to September 28, 2016. There was no language restriction. The searching strategy consisted of key terms related to the PEG, dysphagia, and randomized controlled trials (RCTs). Detailed searching strategy in PubMed was shown in Table 1.

Full table
Studies that met the following criteria were considered eligible: (I) RCTs investigating efficacy and safety of PEG; (II) the study was conducted in adult patients with dysphagia. The following citations were excluded: (I) animal and/or experimental studies; (II) observational studies; (III) study setting was in the ward or community; (IV) reviews and commentaries.
If there were systematic reviews in the field, we adopted the results of the most updated systematic review as the evidence. The use of available systematic reviews might help to avoid repeated work, without compromising the quality of the present review. We also tried to identify eligible articles published after the search time period used in the most updated systematic review.
Results
The initial search identified 19 citations and 1 systematic review (7). There are no new RCTs published after that time. As a result, we adopted this systematic review as the most updated evidence.
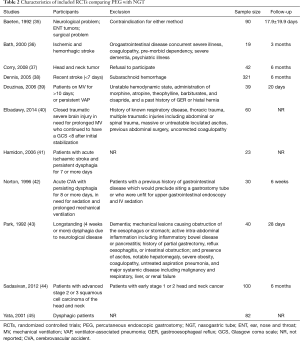
A total of 11 RCTs were included in the systematic review (Table 2) (35-45). Four trials explicitly stated they included patients with CVA complicated by dysphagia (36,38,41,42). Overall, the intervention failure occurred less frequently in the PEG group than in the NGT group (RR: 0.18; 95% CI: 0.05 to 0.59). However, there was no difference on mortality (RR: 0.86; 95% CI: 0.58 to 1.28), or aspiration pneumonia (RR: 0.70; 95% CI: 0.46 to 1.06). With respect to nutritional status, PEG was able to improve mid-arm circumference (MD: 1.16; 95% CI: 1.01 to 1.31) and level of serum albumin (MD: 6.03; 95% CI: 2.31 to 9.74). The intervention favored PEG over NGT on quality of life measures (EuroQol, RR: 0.03; 95% CI: 0.00 to 0.29), discomfort (RR: 0.03; 95% CI: 0.00 to 0.29), altered body image (RR: 0.01; 95% CI: 0.00 to 0.18) and social activities (RR: 0.01; 95% CI: 0.00 to 0.18).
Full table
Comments
Malnutrition is common among patients post-CVA, and has been shown to be an independent predictor of poor outcomes (46-48). Thus the prompt initiation of enteral nutrition is of paramount importance. Current evidence and practice favors PEG over NGT placement for patients with swallowing disturbances. Although some major outcomes such as mortality, aspiration pneumonia cannot be reduced (7). Placement of a PEG tube has also been show to improve the nutritional status and quality of life in patient with chronic aspiration secondary to dysphagia (49). The timing of PEG insertion is another important issue that requires consideration. Several guidelines have recommended PEG placement (50-53), but there is no specific recommendation regarding the timing of PEG placement. Although PEG placement is considered a low risk procedure, PEG placement at acute phase is associated with worse outcomes in comparison to NGT (54,55). Thus, in our experience 3 or 4 weeks can be allowed for the recovery of swallowing ability before considering PEG.
Evidence for TT placement
Search strategy and study selection
Electronic database of PubMed was searched from inception to September 28, 2016. There was no language restriction. The search strategy consisted of key terms related to the tracheostomy and RCTs.
Studies that met the following criteria were considered eligible: (I) RCTs investigating the timing of tracheostomy; (II) the study was conducted in adult patients requiring MV and/or airway protection. The following citations were excluded: (I) animal and/or experimental studies; (II) observational studies; (III) study setting was in the ward or community; (IV) reviews and commentaries.
If there were systematic reviews in the field, we adopted the results of the most up-to-date systematic review as the evidence. We also tried to identify eligible articles published after the search time period used in the most updated systematic review.
Results
The initial search identified 307 citations. We identified one systematic review from Cochrane database which focused on the timing of tracheostomy in the critically ill (56).
In critically ill patients, Andriolo and colleagues identified eight RCTs (56). They defined ET as 2 to 10 days after intubation, and late tracheostomy (LT) was defined as >10 days after intubation. The pooled results showed that ET as compared with the late group had lower risk of death (RR: 0.83; 95% CI: 0.70 to 0.98; P=0.03). The number needed to treat for an additional beneficial outcome (NNTB) was around 11. Also, the probability of discharge from the ICU was higher at day 28 in the ET group (RR: 1.29; 95% CI: 1.08 to 1.55; P=0.006; NNTB ≈8). In systematic review restricting to patients with acute brain injury, McCredie VA employed the same definitions of early and LT and found that ET reduced long-term mortality (RR: 0.57; 95% CI: 0.36 to 0.90; P=0.02). For other secondary outcomes, ET reduced ICU length of stay (MD: −2.55 days; 95% CI: −0.50 to −4.59; P=0.01; n=326) and duration of MV (MD: −2.72 days; 95% CI: −1.29 to −4.15; P=0.0002; n=412) (14). However, the timing (early versus late) TT placement is controversial. There is evidence from systematic reviews that ET had no significant effect in clinical outcomes compared to that of the LT/prolonged intubation (PI) group (57,58). Others suggested that ET may shorten the duration of sedation (17). In patients with subarachnoid hemorrhage ET was associated with fewer respiratory adverse events (59). Given the conflicting results of current studies, more investigations are needed, with standard definition of timing and homogeneous study population.
Comments
The beneficial effects of ET versus LT are controversial. There is evidence suggesting that early (<10 days) tracheostomy is beneficial for patients intubated and expected need of MV for at least 2 weeks (60). However, others showed no significant effect in clinical outcomes with ET versus LT. For some elderly stroke patients, they may not need MV but tracheostomy is required to keep a patent airway.
Conclusions
Approximately 10% of the patients requiring long-term MV may eventually undergo tracheostomy (61-63), with slightly more than half also needing PEG placement for enteral nutrition (64). Although there is evidence that early PEG placement is associated with increased risk of death, for a subgroup of patients with recurrent aspiration pneumonia after stroke the use of the PENlIGhT procedure may not only aid in providing adequate enteral nutrition but also in the suctioning of airway secretions. For some patients with dismal neurological outcome, the family members can never make a decision to stop treatment. The PENlIGhT protocol may be able to aid in expediting patient transfer to other levels of care (65). Some experts proposed that placement of both PEG and TTs at the same time had the potential for decreased costs, anesthesia exposure, procedural times, ventilator times, and ICU days (64). That said, the PENlIGhT protocol has not been validated by well-designed RCTs, and requires further investigation (66). Selection of appropriate patients is the core to making the PENlIGhT protocol clinically useful. Patients should be expected to have prolonged swallowing disturbance and mechanical ventilation. Some prediction tools can be helpful to make clinical decision and consultation. Patients with poor neurological outcome who require prolonged maintenance of life are good candidates for the PENlIGhT procedure.
Acknowledgements
Funding: The study was supported by Zhejiang Medical Science and Technology projects (2015117919) and Zhejiang Provincial Science and technology projects (2013C33G2010401).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: The funder has no role in the design and conduct of the review.
References
- Bernhardt J, Godecke E, Johnson L, et al. Early rehabilitation after stroke. Curr Opin Neurol 2017;30:48-54. [Crossref] [PubMed]
- Paranji S, Paranji N, Wright S, et al. A Nationwide Study of the Impact of Dysphagia on Hospital Outcomes Among Patients With Dementia. Am J Alzheimers Dis Other Demen 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005;36:2756-63. [Crossref] [PubMed]
- Rodrigue N, Côté R, Kirsch C, et al. Meeting the nutritional needs of patients with severe dysphagia following a stroke: an interdisciplinary approach. Axone 2002;23:31-7. [PubMed]
- Ehsaan F, Ghayas Khan MS, Malik SN, et al. Frequency of post-stroke dysphagia in Pakistan: a hospital based study. J Pak Med Assoc 2016;66:1281-5. [PubMed]
- Martin RE. Neuroplasticity and swallowing. Dysphagia 2009;24:218-29. [Crossref] [PubMed]
- Gomes CA Jr, Andriolo RB, Bennett C, et al. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst Rev 2015.CD008096. [PubMed]
- Walcott BP, Kamel H, Castro B, et al. Tracheostomy after severe ischemic stroke: a population-based study. J Stroke Cerebrovasc Dis 2014;23:1024-9. [Crossref] [PubMed]
- Seder DB, Jagoda A, Riggs B. Emergency Neurological Life Support: Airway, Ventilation, and Sedation. Neurocrit Care 2015;23 Suppl 2:S5-22. [Crossref] [PubMed]
- Miller CM, Pineda J, Corry M, et al. Emergency Neurologic Life Support (ENLS): Evolution of Management in the First Hour of a Neurological Emergency. Neurocrit Care 2015;23 Suppl 2:S1-4. [Crossref] [PubMed]
- Schönenberger S, Niesen WD, Fuhrer H, et al. Early tracheostomy in ventilated stroke patients: Study protocol of the international multicentre randomized trial SETPOINT2 (Stroke-related Early Tracheostomy vs. Prolonged Orotracheal Intubation in Neurocritical care Trial 2). Int J Stroke 2016;11:368-79. [Crossref] [PubMed]
- Al Sharhan S, Sohail M, Ahmad K, et al. Self-reported comfort with tracheostomy tube care. Cross-sectional survey of non-ear, nose and throat health care professionals. Saudi Med J 2014;35:63-6. [PubMed]
- Terragni P, Faggiano C, Martin EL, et al. Tracheostomy in mechanical ventilation. Semin Respir Crit Care Med 2014;35:482-91. [Crossref] [PubMed]
- McCredie VA, Alali AS, Scales DC, et al. Effect of Early Versus Late Tracheostomy or Prolonged Intubation in Critically Ill Patients with Acute Brain Injury: A Systematic Review and Meta-Analysis. Neurocrit Care 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med 2016;44:390-438. [Crossref] [PubMed]
- Zhang Z, Li Q, Jiang L, et al. Effectiveness of enteral feeding protocol on clinical outcomes in critically ill patients: a study protocol for before-and-after design. Ann Transl Med 2016;4:308. [Crossref] [PubMed]
- Meng L, Wang C, Li J, et al. Early vs late tracheostomy in critically ill patients: a systematic review and meta-analysis. Clin Respir J 2016;10:684-692. [Crossref] [PubMed]
- Vaughan JR, Scott JS, Edelman DS, et al. Tracheostomy. A new indication for percutaneous endoscopic gastrostomy tube placement. Am Surg 1991;57:214-5. [PubMed]
- Slezak FA, Kofol WH. Combined tracheostomy and percutaneous endoscopic gastrostomy. Am J Surg 1987;154:271-3. [Crossref] [PubMed]
- Belanger A, Akulian J. Interventional pulmonology in the intensive care unit: percutaneous tracheostomy and gastrostomy. Semin Respir Crit Care Med 2014;35:744-50. [Crossref] [PubMed]
- D'Amelio LF, Hammond JS, Spain DA, et al. Tracheostomy and percutaneous endoscopic gastrostomy in the management of the head-injured trauma patient. Am Surg 1994;60:180-5. [PubMed]
- Trapl M, Enderle P, Nowotny M, et al. Dysphagia bedside screening for acute-stroke patients: the Gugging Swallowing Screen. Stroke 2007;38:2948-52. [Crossref] [PubMed]
- Daniels SK, Anderson JA, Willson PC. Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke 2012;43:892-7. [Crossref] [PubMed]
- Daniels SK, Ballo LA, Mahoney MC, et al. Clinical predictors of dysphagia and aspiration risk: outcome measures in acute stroke patients. Arch Phys Med Rehabil 2000;81:1030-3. [Crossref] [PubMed]
- Daniels SK, Brailey K, Foundas AL. Lingual discoordination and dysphagia following acute stroke: analyses of lesion localization. Dysphagia 1999;14:85-92. [Crossref] [PubMed]
- Addington WR, Stephens RE, Gilliland K, et al. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil 1999;80:150-4. [Crossref] [PubMed]
- Mandaville A, Ray A, Robertson H, et al. A retrospective review of swallow dysfunction in patients with severe traumatic brain injury. Dysphagia 2014;29:310-8. [Crossref] [PubMed]
- Faigle R, Marsh EB, Llinas RH, et al. Novel score predicting gastrostomy tube placement in intracerebral hemorrhage. Stroke 2015;46:31-6. [Crossref] [PubMed]
- Dubin PH, Boehme AK, Siegler JE, et al. New model for predicting surgical feeding tube placement in patients with an acute stroke event. Stroke 2013;44:3232-4. [Crossref] [PubMed]
- Wermker K, Jung S, Hüppmeier L, et al. Prediction model for early percutaneous endoscopic gastrostomy (PEG) in head and neck cancer treatment. Oral Oncol 2012;48:355-60. [Crossref] [PubMed]
- Kumar S, Langmore S, Goddeau RP Jr, et al. Predictors of percutaneous endoscopic gastrostomy tube placement in patients with severe dysphagia from an acute-subacute hemispheric infarction. J Stroke Cerebrovasc Dis 2012;21:114-20. [Crossref] [PubMed]
- Kollef MH, Ahrens TS, Shannon W. Clinical predictors and outcomes for patients requiring tracheostomy in the intensive care unit. Crit Care Med 1999;27:1714-20. [Crossref] [PubMed]
- Yaghi S, Moore P, Ray B, et al. Predictors of tracheostomy in patients with spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg 2013;115:695-8. [Crossref] [PubMed]
- Szeder V, Ortega-Gutierrez S, Ziai W, et al. The TRACH score: clinical and radiological predictors of tracheostomy in supratentorial spontaneous intracerebral hemorrhage. Neurocrit Care 2010;13:40-6. [Crossref] [PubMed]
- Baeten C, Hoefnagels J. Feeding via nasogastric tube or percutaneous endoscopic gastrostomy. A comparison. Scand J Gastroenterol Suppl 1992;194:95-8. [Crossref] [PubMed]
- Bath PM, Bath FJ, Smithard DG. Interventions for dysphagia in acute stroke. Cochrane Database Syst Rev 2000.CD000323. [PubMed]
- Corry J, Poon W, McPhee N, et al. Randomized study of percutaneous endoscopic gastrostomy versus nasogastric tubes for enteral feeding in head and neck cancer patients treated with (chemo)radiation. J Med Imaging Radiat Oncol 2008;52:503-10. [Crossref] [PubMed]
- Dennis MS, Lewis SC, Warlow C, et al. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet 2005;365:764-72. [Crossref] [PubMed]
- Douzinas EE, Tsapalos A, Dimitrakopoulos A, et al. Effect of percutaneous endoscopic gastrostomy on gastro-esophageal reflux in mechanically-ventilated patients. World J Gastroenterol 2006;12:114-8. [Crossref] [PubMed]
- Elbadawy TH, Gamal MA, Fayed AM, et al. S123. Early gastrostomy and tracheostomy prevent ventilator associated pneumonia in traumatic brain injured patients. European Society of Intensive Care Medicine (ESICM) Congress, Springer Verlag, 13-17 October, 2012.
- Hamidon BB, Abdullah SA, Zawawi MF, et al. A prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with acute dysphagic stroke. Med J Malaysia 2006;61:59-66. [PubMed]
- Norton B, Homer-Ward M, Donnelly MT, et al. A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ 1996;312:13-6. [Crossref] [PubMed]
- Park RH, Allison MC, Lang J, et al. Randomised comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with persisting neurological dysphagia. BMJ 1992;304:1406-9. [Crossref] [PubMed]
- Sadasivan A, Faizal B, Kumar M. Nasogastric and percutaneous endoscopic gastrostomy tube use in advanced head and neck cancer patients: a comparative study. J Pain Palliat Care Pharmacother 2012;26:226-32. [Crossref] [PubMed]
- Yata M, Date K, Miyoshi H, et al. Comparison between nasogastric tube feeding and percutaneous endoscopic gastrostomy feeding a long-term randomized controlled study. Gastrointestinal Endoscopy 2001;53:AB206.
- Gariballa SE, Parker SG, Taub N, et al. A randomized, controlled, a single-blind trial of nutritional supplementation after acute stroke. JPEN J Parenter Enteral Nutr 1998;22:315-9. [Crossref] [PubMed]
- FOOD Trial Collaboration. Poor nutritional status on admission predicts poor outcomes after stroke: observational data from the FOOD trial. Stroke 2003;34:1450-6. [Crossref] [PubMed]
- Finestone HM, Greene-Finestone LS, Wilson ES, et al. Prolonged length of stay and reduced functional improvement rate in malnourished stroke rehabilitation patients. Arch Phys Med Rehabil 1996;77:340-5. [Crossref] [PubMed]
- Hossein SM, Leili M, Hossein AM. Acceptability and outcomes of percutaneous endoscopic gastrostomy (PEG) tube placement and patient quality of life. Turk J Gastroenterol 2011;22:128-33. [Crossref] [PubMed]
- Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870-947. [Crossref] [PubMed]
- Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:3020-35. [Crossref] [PubMed]
- National Collaborating Centre for Acute Care (UK). Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition. London: National Collaborating Centre for Acute Care (UK), 2006.
- Wirth R, Smoliner C, Jäger M, et al. Guideline clinical nutrition in patients with stroke. Exp Transl Stroke Med 2013;5:14. [Crossref] [PubMed]
- Dennis M, Lewis S, Cranswick G, et al. FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke. Health Technol Assess 2006;10:iii-iv, ix-x, 1-120.
- Janes SE, Price CS, Khan S. Percutaneous endoscopic gastrostomy: 30-day mortality trends and risk factors. J Postgrad Med 2005;51:23-8; discussion 28-9. [PubMed]
- Andriolo BN, Andriolo RB, Saconato H, et al. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev 2015;1:CD007271. [PubMed]
- Huang H, Li Y, Ariani F, et al. Timing of tracheostomy in critically ill patients: a meta-analysis. PLoS One 2014;9:e92981. [Crossref] [PubMed]
- Wang F, Wu Y, Bo L, et al. The timing of tracheotomy in critically ill patients undergoing mechanical ventilation: a systematic review and meta-analysis of randomized controlled trials. Chest 2011;140:1456-65. [Crossref] [PubMed]
- Gessler F, Mutlak H, Lamb S, et al. The Impact of Tracheostomy Timing on Clinical Outcome and Adverse Events in Poor-Grade Subarachnoid Hemorrhage. Crit Care Med 2015;43:2429-38. [Crossref] [PubMed]
- Bösel J, Schiller P, Hook Y, et al. Stroke-related Early Tracheostomy versus Prolonged Orotracheal Intubation in Neurocritical Care Trial (SETPOINT): a randomized pilot trial. Stroke 2013;44:21-8. [Crossref] [PubMed]
- Fischler L, Erhart S, Kleger GR, et al. Prevalence of tracheostomy in ICU patients. A nation-wide survey in Switzerland. Intensive Care Med 2000;26:1428-33. [Crossref] [PubMed]
- Frutos-Vivar F, Esteban A, Apezteguía C, et al. Outcome of mechanically ventilated patients who require a tracheostomy. Crit Care Med 2005;33:290-8. [Crossref] [PubMed]
- Freeman BD, Borecki IB, Coopersmith CM, et al. Relationship between tracheostomy timing and duration of mechanical ventilation in critically ill patients. Crit Care Med 2005;33:2513-20. [Crossref] [PubMed]
- Yarmus L, Gilbert C, Lechtzin N, et al. Safety and feasibility of interventional pulmonologists performing bedside percutaneous endoscopic gastrostomy tube placement. Chest 2013;144:436-40. [Crossref] [PubMed]
- Wall A. Immortalization: placement of a percutaneous endoscopic gastrostomy tube and tracheostomy in a neurologically devastated patient. Narrat Inq Bioeth 2015;5:25-8. [Crossref] [PubMed]
- Slattery E, Seres DS. Just because we can does not mean we should: a perspective on combined tracheostomy and percutaneous endoscopic gastrostomy tube insertion. Chest 2014;145:421-2. [Crossref] [PubMed]


