The role of bronchoscopy in the diagnosis of airway disease
Introduction
Bronchoscopy has come a long way since it was first described in 1897 by Gustav Killian, who had used it to remove a pork bone from a farmer’s airway (1,2). Since that time, bronchoscopy has evolved to become an integral tool for thoracic surgeons both for evaluating airway and lung pathology and for therapeutic interventions. The modern rigid bronchoscope was first described by Philadelphia based otolaryngologist Chevalier Jackson in 1904 (3). Although it is currently used primarily for therapeutic interventions, it is still a valuable diagnostic instrument and a skill that physicians should be familiar with. Flexible bronchoscopy was first used in 1967 and with technological advancement the development of endobronchial ultrasound (EBUS) and electromagnetic navigational bronchoscopy (ENB) have provided endoscopic tools to examine and biopsy mediastinal and peripheral pulmonary lesions (PPL) (1). This review will focus on the role of bronchoscopy, including EBUS, ENB, and rigid bronchoscopy (RB), in the diagnosis of bronchopulmonary diseases. In addition, it will cover the anesthetic considerations, equipment, diagnostic yield, and potential complications.
Anesthetic considerations for bronchoscopy
Maintaining the airway is critically important while performing a procedure that instruments the airway passages for direct viewing or interventional procedure. If there are separate anesthetic provider and proceduralists, there is significant co-management of the airway, and therefore close communication is critical. During induction, the bronchoscopist should be present and ready to establish emergency airway access as induction of anesthesia may lead to loss of a previously patent airway (4). If this were to occur, RB or a surgical airway access should be performed if standard measures to establish endotracheal intubation are unsuccessful. This will be particularly important in cases involving airway foreign bodies and mediastinal masses. Aside from these considerations, anesthetic management for flexible bronchoscopy and RB can vary significantly.
Flexible bronchoscopy can be performed under sedation or general anesthesia. Relative contraindications to sedation include history of severe gastroesophageal reflux, history of aspiration, respiratory compromise, and extreme anxiety. Sedation can be accomplished in a number of ways including any combination of propofol or dexmedetomidine infusion, midazolam, and fentanyl. Ketamine is less commonly used due to its propensity to increase airway secretions and cause hallucinations (5). Topical anesthesia is useful as well with lidocaine being the anesthetic of choice (6). Strategies for topical anesthesia include transtracheal injection, nebulized solutions, and topical application to the posterior pharynx either by having the patient gargle viscous lidocaine or by direct spray to the mucosa of the larynx and trachea (5-7). If general anesthesia with an endotracheal tube (ETT) is required, a large ETT is ideal as the presence of the flexible bronchoscope in the ETT significantly reduces airway diameter and increases airway resistance. For an adult flexible bronchoscope, the minimum size ETT should be 8.0 mm internal diameter. Using a smaller ETT may lead to intrinsic PEEP and dynamic hyperinflation (5-7). When not contraindicated, a laryngeal mask airway (LMA) should be considered as it has a larger diameter conduit for the flexible bronchoscope thus reducing airway pressures. In addition, it allows for complete visualization of the trachea from the vocal cords whereas the ETT has to be extracted to view the proximal trachea and vocal cords (6).
Rigid bronchoscopy requires general anesthesia and almost always paralysis. A total intravenous anesthetic (TIVA) with propofol is the most common technique for maintenance of anesthesia. Remifentanil is an ideal adjunct for a TIVA as it tends to provide dense, short acting, and predictable analgesia (6,7). Short acting, non-depolarizing agents or a succinylcholine drip are preferred (5,7). Many different ventilatory strategies have been successfully used. The simplest technique is to use the standard semi-closed circuit by connecting the ventilator circuit to the side port of the rigid bronchoscope, essentially treating the bronchoscope like an ETT. Ventilation is held whenever the eyepiece is removed from the proximal end for suction or biopsy. Additionally, if a telescope is passed through the rigid scope, ventilation through the side piece via the standard ventilator circuit will not be possible. While an inhaled agent can be used with this system, delivery may be hampered by frequent pauses in ventilation and due to suctioning. Additionally, high flows (up to 20 L/min) may be required to compensate for leaks in the system, which leads to inefficient delivery of the inhaled anesthetic and leakage of gas into the operating room (4,7,8). Saline soaked gauze can be packed in the posterior oropharynx to help reduce the leak. A Jackson-Reese circuit can also be connected to the side port of the rigid bronchoscope with intermittent volume ventilation performed by squeezing the bag whenever the scope is free of a telescope. Again, the proximal end must be occluded with the eyepiece to allow for ventilation to occur. Because a large portion of the time RB is performed for intervention, and the working channel needs to be open, the authors prefer jet ventilation. Jet ventilation uses an injector bronchoscope, however, some find it undesirable as it can create noise and aerosolized secretions (4). Additionally, patients are at risk for barotrauma and hypercarbia with this technique (6,8). With jet ventilation, it is critical to keep the end of the bronchoscope open to avoid accumulation of pressure and barotrauma (9). Apneic oxygenation can be performed as well using a small catheter positioned alongside the bronchoscope to insufflate oxygen, but this technique is rarely used due to the propensity for significant hypercarbia.
Flexible bronchoscopy
Flexible bronchoscopy is simple to learn and provides many benefits as a diagnostic, but also a therapeutic treatment option for patients with bronchopulmonary diseases. Flexible bronchoscopes have a fiber-optic light source that illuminates the distal end of the scope allowing for visualization of the airways. A suction port allows for aspiration of fluid and de-fogging of the camera through a working channel. A single forward and backward toggle bends the tip of the scope 120–180 degrees, and when combined with wrist rotation allows access from the trachea to the quaternary airways.
Flexible bronchoscopes come in many different variations, but generally there are three size categories: pediatric, adult, and therapeutic scopes. Pediatric or ultrathin scopes have an outer diameter of 2.8 mm and working channel width of 1.2 mm. The smaller channel size allows for the passage of small instruments such as cytology brushing and biopsy forceps, as well as, for suctioning and bronchoalveolar lavage (BAL) collection. Pediatric scopes have a more narrow scope body that can allow for navigation around obstructing lesions; however, the image clarity is often limited in scopes of smaller sizes.
The larger typical adult scopes have an outer diameter of 4.9–5.5 mm and a working channel size of 2.0 mm. This size working port allows for biopsy forceps or needles, baskets, and greater suctioning abilities, as well as, some other diagnostic and therapeutic adjuvants. Therapeutic bronchoscopes have the largest working channel width (2.8–3.2 mm) and outer diameter of 6.0–6.2 mm. The larger working channel (>3 mm) is needed for laser or electrocautery device insertion.
Older generation fiber-optic bronchoscopes have an eye piece, but most institutions use video-linked bronchoscopes that display the images on video monitors which allow for improved teaching platforms and visualization for multiple operators while performing complex interventions.
The bronchoscopy procedure is very well tolerated and most commonly performed as an outpatient day procedure. Adverse events are rare but include: bleeding (0.12%), hypoxia, loss of airway, pneumothorax (0.1–0.16%), or mortality (0–0.02%) (10-12).
The most common uses for flexible bronchoscopy include: diagnosing potential airway injury or obstructions such as with foreign body or tumor, biopsy of airway masses for diagnosis, specific guided endobronchial washings for diagnosis of micro-bacterial evaluation, or pulmonary hygiene for pulmonary lobar collapse. Visual inspection of the airway is the simplest diagnostic utility with flexible bronchoscopy. The airway is evaluated for possible intraluminal irregularity, obstruction, foreign body, fluids and mucous, inhalation injury, caustic ingestion, or trauma.
Airway injuries may not be identified on imaging such as chest radiograph or CT scan. Liberal use of bronchoscopy in blunt or penetrating trauma aids in the diagnosis of occult airway injuries, which is important because patient outcomes were improved with early diagnosis (13,14). Inhalation burn injury leads to hyperemia in the airways of burn patients that can be visualized on flexible bronchoscopy. Bronchoscopic diagnosis of airway injury is associated with increased mortality after inhalation injuries and therefore prompt diagnosis and management is extremely important (15).
Characteristic chest X-ray findings of airway obstruction include opacification of the lung parenchyma and tracheal deviation towards the side of collapse. However, often times it is difficult to distinguish lobar collapse from a large pulmonary effusion. Flexible bronchoscopy is an excellent diagnostic tool as well as a potentially therapeutic technique to clear mucous plugging causing lobar collapse. The procedure requires a standard (adult) size bronchoscope to fully suction thick secretions. Sterile saline is flushed through the working port in order to break up thick secretions. Culture samples should be sent in order to appropriately treat bacterial pneumonia. Bronchoscopy washings will allow for investigation of infectious pulmonary disease by obtaining culture material in order to better treat patients with targeted antimicrobials. Blind catheter aspiration should be obtained first for ventilated patient with suspected ventilator associated pneumonia, but if non-diagnostic then bronchoscopy with BAL should be considered for diagnosis (10).
Masses within the tracheobronchial tree can also lead to airway obstruction and lobar parenchymal collapse. Suspected mass evaluation via flexible bronchoscopy allows for differentiation between tumors and aspirated foreign bodies. It may also obviate the need for RB, if the foreign body can be retrieved via forceps, basket, suction, snare, or cryotherapy (16,17). Tumors are evaluated for extent of involvement by visual inspection and should be biopsied for pathologic diagnosis. For patients with suspected lung cancer, the diagnostic yield of flexible bronchoscopy alone is high (74%) when there is an endobronchial component that is visible and forceps biopsy is performed (10). The yield is increased with the addition of endoscopic brushings (18) (Figure 1).
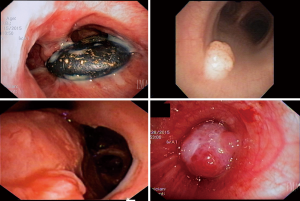
Patients with airway bleeding can develop blood clots that result in bronchial obstruction. Flexible bronchoscopy can be used for evacuation of blood from the airway; however large clots often require RB for complete evacuation. Flexible bronchoscopy allows for other adjunctive treatments including cryoablation or argon plasma coagulation of bleeding lesions (19,20).
Biopsies of alveolar tissue via flexible bronchoscopy are useful in diagnosing many interstitial lung diseases including sarcoidosis, non-interstitial pneumonia, idiopathic pulmonary fibrosis, or rejection after lung transplant. Traditionally, parenchymal biopsies were obtained with flexible forceps that grasp the tissue and extract it through the working channel. However, a newer innovation cryoprobe transbronchial lung biopsy can aid in diagnosis. As the cryoprobe is activated, the tip adheres to the tissue due to crystallization of water and can thus be extracted (21).
The Wang needle was developed in 1978 and was first used to diagnose lung cancers. It was then further used to perform transbronchial biopsies of mediastinal lymph nodes. This was historically done using landmarks and anatomic knowledge in a blind fashion (22). Now this is largely replaced by EBUS with biopsy as discussed below.
EBUS
The technology of EBUS has evolved significantly since its birth over a decade ago. As endoscopists gained experience with EBUS, there has been an increase in the diagnostic uses and an improved yield with biopsies. EBUS probes combine bronchoscopy with an ultrasound probe at the distal end for viewing extra bronchial structures including mediastinal vascular structures, masses, or lymph nodes. The scopes have a working channel for trans-bronchial fine needle aspiration (TBNA).
There are two main types of EBUS scopes available: radial probe and convex probe EBUS. The radial probe (UM-S320-20R, Olympus), which was developed first, does not allow for direct visualization during biopsy. It requires the probe to be placed through a guide sheath and then both are advanced through the working channel of a regular bronchoscope. After identification of the lesion of interest, the probe is removed and the working sheath remains in place and biopsy forceps or brushing wand may be inserted through the sheath for sampling (23). The convex probe EBUS (XBF-UC260F-OL8, Olympus) is an integrated scope and transducer that allows for direct visualization during FNA biopsy (23). The balloon tipped scope allows for saline insufflation to aid in ultrasound transduction and clearer ultrasound imaging. A 21- or 22-gauge needle is inserted with sheath through the working port for TBNA while directly visualizing the biopsy target of interest. The needle extends out of the working channel into the node or lesion with the ability to apply a suction syringe to the opposing end. The needle is repeatedly passed within the tissue under direct visualization to obtain the samples. The entire needle apparatus is then removed and the tissue placed onto a slide for rapid on site evaluation (ROSE) or into a cytology container. Three to five passes of the needle is recommended for optimal diagnostic potential (24).
EBUS, like bronchoscopy, can be performed under conscious sedation with local anesthetic to the vocal cords or general anesthesia through an ETT. Complication rates are low (0–3%) and include: hypoxia, respiratory failure, pneumomediastinum, pneumothorax, cardiac complications, and bleeding. Rarely sepsis and airway injury have been reported (25-28). Lung nodules are often alternatively sampled via CT guided percutaneous biopsies, which can lead to a higher rate of pneumothorax or biopsy tract seeding. In conventional series, CT guided biopsy has a pneumothorax rate of 15%, with 40% of these patients requiring chest tube placement (29).
EBUS-TBNA is quickly becoming the standard for diagnosis of pathologic or enlarged mediastinal lymph nodes. Needle aspiration samples are conducive to immediate pathologic evaluation and allow potential concomitant surgical resection in the appropriate settings. Lung cancer staging is the most common indication for mediastinal lymph node analysis, but EBUS is used for non-malignant pathologies as well.
EBUS evaluation of mediastinal lymph nodes for lung cancer aids in diagnosing advanced disease and allows for preoperative planning. Gold standard mediastinal sampling has traditionally been done surgically via mediastinoscopy, an invasive surgical procedure with a cervical incision. However, a recent study showed that EBUS-TBNA was more sensitive (88% vs. 91%) and more accurate (92% vs. 89%) when compared to mediastinoscopy for evaluating lymph node stations 2R, 2L, 4R, 4L, and 7 (30). The European Society of Thoracic Surgeons have thus changed their guidelines to recommend EBUS-TBNA as first line method of mediastinal lymph node assessment in patients with non-small cell lung cancers greater than 3 cm, located centrally within the lung, or with suspected N2 disease based on pre-procedure imaging (31).
Another advantage EBUS has over mediastinoscopy is the ability to sample N1 level lymph nodes (stations 10 and 11) and intrapulmonary or parenchymal lesions. Lung lesions near the airway, but not directly visualized by bronchoscopy have a diagnostic accuracy up to 94% (25). When comparing blind trans-bronchial biopsy to EBUS-TBNA, there was no difference in lesions greater than 3 cm. But with lesions smaller than 3 cm adding EBUS imaging significantly increased the trans-bronchial biopsy rate from 31% to 75% (32). Unfortunately, the sensitivity of EBUS decreases with sampling of more distal PPL. In a retrospective analysis of patients with PPL’s, EBUS was found to have 60% sensitivity in establishing a lung cancer diagnosis in lesions seen on ultrasound. When including lesions not visualized, the sensitivity dropped to 49% (33). Georgiou et al. found similar results but also noted a higher sensitivity of 85–87% when lesions were >20 mm and an overall sensitivity of 69.7% in detecting PPL’s (34). Factors that increased the diagnostic yield of EBUS for PPL’s include: bronchus sign on CT, solid nodules, lesion >20 mm, and probe position within the lesion (35). A bronchus sign describes when CT imaging demonstrates a bronchus headed directly towards the lesion of interest. Radial probe EBUS with ROSE pathologic evaluation will also increase diagnostic accuracy of PPL’s (36). Endoscopic ultrasound guided FNA (through the esophagus) can increase access to left paratracheal, inferior mediastinum, and aortopulmonary window nodes. When added to EBUS-TBNA, it can increase the ability to diagnose lymphadenopathy by 7.6% (37).
Non-lung cancer pathologies investigated by EBUS include: lymphoma, sarcoidosis, or tuberculosis. In a recent retrospective analysis, the overall sensitivity and negative predictive value of EBUS-TBNA in diagnosing lymphoma were 65% and 96%, respectively (38). However, it is important to note that other studies over the last 8-year have found wide variability in the sensitivity of EBUS-TBNA for lymphoma (39-45). Most studies are limited by small sample sizes and differences in disease recurrence, as well as mixtures of patients with Hodgkins lymphoma (HL) and non-Hodgkins lymphoma (NHL). Results showed higher diagnostic sensitivity with NHL and those with recurrent disease (38).
A recent study looking at the use of EBUS-TBNA for diagnosis for sarcoidosis found an overall sensitivity of 84%. Additionally, they note that when combined with standard bronchoscopic techniques, sensitivity increases to 89%. This is compared to a sensitivity of 54% with standard bronchoscopic techniques alone (46). A systemic review and meta-analysis of EBUS-TBNA for diagnosis for sarcoidosis found an overall diagnostic accuracy of 79% (47).
EBUS-TBNA is an effective means of diagnosis of intrathoracic tuberculosis. A recent review of eight studies with 809 patients found a pooled sensitivity and specificity of 80% and 100%, respectively. This is most helpful in cases of lymphadenopathy with negative microbiologic sputum cultures (48).
As discussed earlier, flexible bronchoscopy has been used to visually inspect and evaluate malignant spread through the bronchial wall and into the lumen of the airway, which can help in clinical management and treatment options. EBUS is now being used to distinguish between compression and infiltration of the bronchial wall. Use of ultrasound to evaluate the extent of tumor involvement has previously been useful for esophageal and rectal cancer staging. Within the tracheal wall, ultrasound has an accuracy of 94% for determining tumor involvement, when compared to the resected specimens. This is much higher than CT image accuracy of 51% (49). This technology is useful for not only lung cancers, but when considering resection of thyroid or esophageal cancers with questionable tracheal involvement.
Navigational bronchoscopy
ENB, or more commonly called navigational bronchoscopy, became commercially available in 2006 and combines CT images with real time bronchoscopy that allows for biopsy of thoracic and pulmonary lesions. It was developed to allow for trans-bronchial biopsy of more remote peripheral lesions than those available for biopsy with traditional bronchoscopy or EBUS (50).
The technology requires iLogic virtual bronchoscopy proprietary planning software and equipment (SuperDimensionTM, Medtronic) (51). The system contains three components: dedicated laptop with planning software, the electromagnet mat, and a freestanding video tower. There are two phases of the procedure: the planning stage and procedure stage.
During the planning stage, a dedicated thin slice CT scan of the patient is obtained. This is then loaded into the dedicated laptop with software that identifies airways within the lung, and it renders a three-dimensional representation of the bronchial tree. A physician then must direct the computer to finalize the lesion mapping. The lesion is outlined and identified to the computer. A pathway or “road map” similar to the color-highlighted roadway maps on conventional global positioning systems (GPS) is created that leads to the lesion. Several pathway maps to a single nodule can be stored in the computer to increase the rate of a successful diagnostic biopsy.
The patient is then taken to the procedure suite. The electromagnetic mat is placed under the patient’s chest and then the bronchoscope is introduced into the airways to allow registration of the mat to the computer-generated airways based on CT scan mapping. A therapeutic bronchoscope with a 3.8-mm working channel is fitted with a steerable locatable guide that registers with the mat under the patient, which is then registered with the three dimensional reconstruction and pathways done during the planning stage (Figure 2). The location guide can extend out the end of the bronchoscope for biopsy out into more distal airways, reaching up to 6th to 8th generation airways (52). ENB is also used to mark peripheral lesions by injecting dye for operative identification or placement of fiducial markers for directed radiation treatment or surgical identification of a small nodule (53).
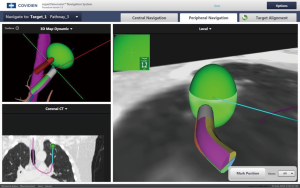
A disadvantage of this procedure is that it can only be performed after obtaining a dedicated CT scan with added radiation exposure to the patient. The additional CT scan, cost of software and a dedicated laptop computer increase the cost of this technology when compared to CT-guided biopsy (54). However, there are fewer complications than with CT-guided biopsy including a reported pneumothorax rate of only 1% (55).
Factors which contribute to the overall diagnostic accuracy of ENB include: the presence of a bronchus sign on CT, location and size of the lesion, general anesthesia versus sedation, and the availability of pathologic ROSE (56,57). The presence of a bronchus sign significantly increases the yield of ENB biopsy compared to no discernable presence of an airway leading to the nodule (79% vs. 31%) (58). Not surprisingly the diagnostic yield of ENB is higher with larger nodules. When lesions are less than or greater than two centimeters, the diagnostic yield is 61% and 83%, respectively (53). However, even with smaller lesions (<15 mm) the diagnostic success rate of biopsy with ENB has been reported to be nearly 70% (59). General anesthesia was found to improve diagnostic yield slightly (69.2% vs. 57.5%) (57). When combined with ROSE, Karnak et al. found ENB to have a pooled diagnostic yield of 89.5% for both mediastinal and peripheral lesions (60).
While one of ENB’s main indications is evaluation of PPL, ENB can also be used for evaluation of mediastinal lymphadenopathy. Diken et al. found ENB to be superior to conventional TBNA in the diagnosis of mediastinal lymphadenopathy. The overall diagnostic yield of ENB when used for diagnosis of mediastinal lymphadenopathy was 72.8% compared to 42.2% for conventional TBNA (59).
Rigid bronchoscopy
Rigid bronchoscopy is a useful tool for an interventional bronchoscopist. The instruments are not complex and have been used for numerous years. However, there are nuances that that must be learned in order for a safe procedure, therefore this procedure should only be performed by those with experience and training in RB.
Rigid bronchoscopy remains the gold standard for the management of many complex airway pathologies. The construction of a rigid bronchoscope is fairly universal consisting of a 40–45 cm straight metal tube that is open on each end. There are side holes along the distal end of the scope, which allow for intermittent ventilation. The distal end is beveled allowing for mechanical resection of obstructing lesions and facilitates opening of the vocal cords during intubation. Various diameter scopes are available including 7, 8, and 9 mm scopes for adults as well as smaller sizes for pediatric patients such as 3.5, 4, 5, and 6 mm. The largest available diameter is 13.5 mm. When choosing a scope size, one should select the largest scope that can safely pass through the glottic opening, which in a male without tight stenosis should be 8–9 mm and in a female 7–8 mm (9). The opposite end of the scope will have multiple ports that can be used for various forms of ventilation including jet ventilation, intermittent volume ventilation, or continuous volume ventilation (9,61).
Rigid bronchoscopy can be performed via direct visualization through the barrel of the scope, or via a rigid optic telescope or video scope passed down through the barrel. Alternatively, some operators will pass a flexible bronchoscope through the barrel. Illumination is accomplished via a light source attached to a light carrier running down the side of the scope or via telescope inserted in the rigid bronchoscope (62). One significant limitation to direct visualization through the barrel is the size of the field of view. The field of view will be 40–45 cm away and relatively small, making visualization of fine details difficult.
Prior to beginning the procedure, inventory of all available equipment should be taken. One should have multiple sizes of rigid bronchoscopes, large bore suction catheters, video telescopes, biopsy forceps, and rubber tooth guard or saline soaked gauze to protect the teeth readily available within the operating room suite. The planned method of ventilation should be discussed with the anesthesia provider ahead of time, as well as an alternative method. The surgeon must be present on induction of anesthesia should there be a need to establish an emergency airway.
For intubation, the patient should be placed in the sniffing position by placing the patient’s head on a pillow and a roll under the shoulders. With the ventilating sidepiece of the scope upward, the scope is inserted into the mouth while protecting the teeth with a mouth piece and the provider’s non-dominant thumb. The scope is directed to the base of the tongue and then elevates the tongue allowing for visualization of the epiglottis, similar to using a laryngoscope. Once the epiglottis is in view, the scope is advanced posteriorly lifting the epiglottis and exposing the glottis. Once the glottis is in view the scope should be rotated 90 degrees to allow it to traverse the vocal cords with its smaller diameter. Once past the glottis, the scope can be rotated back to its original position with the ventilating port directed upwards. To assess the right or left bronchi, the patient’s head is turned to the contralateral side with the scope also placed in the contralateral side of the mouth (9).
Once intubation and visualization are achieved, the large working channel allows the variety of instruments to be used. Forceps extend down into the scope for biopsy, and large suction catheters can be used to aid in visualization and clot removal. Additionally, endobronchial masses can be removed with the beveled end of the rigid bronchoscope by shearing tissue off of the bronchial wall. In the case of obstructing masses, the lesion can be wedged into the distal end of the scope and then the rigid bronchoscope must be withdrawn from the patient for specimen retrieval. The patient is then re-intubated with either rigid bronchoscope or standard ETT. Several passes are often required for large obstructing lesions. Jet ventilation must be held while the specimen is wedged into the end of the scope to ensure it is not expelled out the end. It is our choice to intubate with an ETT upon completion of a RB to allow for ventilation and clearing of carbon dioxide that has built up during high frequency jet ventilation. Finally, the airway is inspected with flexible bronchosocpy for complete resection, airway injury, or bleeding.
The main diagnostic indications for RB include airway foreign bodies, bleeding or hemorrhage into the airway, airway stenosis, evaluation of the airway for tracheal resection, and improved biopsy specimens when fiberoptic specimens are not adequate (9,63). Additionally, examination of the subglottic airway in the neonatal population will usually require RB, especially if any intervention is anticipated (64). RB is often used in high acuity settings, such as an airway foreign body; because once the diagnosis is established it can be used to for therapeutic intervention. Contraindications to RB include uncontrolled coagulopathy, extreme ventilatory and oxygenation demands, facial trauma, and unstable cervical spine (63).
In modern practice, RB is mostly a tool for interventions in the airway. The diagnostic value of RB has decreased with the emergence other diagnostic modalities including flexible bronchoscopy, EBUS, ENB, and high-resolution imaging. These options have become more popular due to the invasive nature of RB. Risks of RB include bronchospasm, hypoxia, and trauma to the respiratory tract including edema or bleeding. A recent review article looking at use of RB for retrieval of airway foreign bodies found that bleeding occurred in 8−17% of cases. In addition, more serious complications including pneumothorax, airway perforation, cardiac arrest, and death have been reported (65).
Rigid bronchoscopy remains the gold standard for extraction of airway foreign bodies. Righini et al suggests initial RB is indicated in cases of asphyxia secondary to obstructive foreign body, radiopaque foreign body on chest X-ray, as well as unilaterally decreased breath sounds, obstructive emphysema or atelectasis on imaging (66). Some have questioned the routine use of RB for airway foreign bodies due to variable rates of negative studies. Various articles have quoted 7–46% negative bronchoscopy rates. Accordingly, recommendations and treatment algorithms have been suggested (65,66). In two recent studies, “obvious” cases of foreign body aspiration went directly to RB, and negative study results were still 14–18% (65,67,68). Since flexible bronchoscopy is a quick and low risk procedure, the authors advocate for a diagnostic bronchoscopy prior to RB if the diagnosis of an aspirated foreign body is in doubt.
Management of bronchopulmonary hemorrhage depends on the rate and quantity of bleeding. A therapeutic flexible bronchoscope will allow for evacuation of majority of airway blood and secretions, as well as allow for airway inspection. When there is extensive hemorrhage, the rigid bronchoscope will accommodate a large bore suction catheter to evacuate rapidly accumulating blood and remove larger formed clots allowing for visualization and identification of the bleeding source (69-71). Once the source is identified, RB affords many different modalities to quickly intervene and control bleeding (70).
RB can be used to evaluate the airway for tracheal resection. Precision is critical when evaluating whether a patient can safely undergo tracheal resection (9,72). Tracheal stenosis is most commonly evaluated by some combination of radiographic imaging and flexible bronchoscopy due to the simplicity and availability of these diagnostic modalities. In this setting, the rigid bronchoscope is used to take precise measurements of the mass or stricture and its distance to the vocal cords and carina. Rigid bronchoscopy is useful if the patient has significant tracheal narrowing in which there is risk for complete occlusion of the airway during the examination. Rigid bronchoscopy is performed with a ventilating instrumentation, which allows the operator to go past the obstruction and ventilate the patient. Furthermore, it is the procedure of choice for dilation, which is likely to be performed in this circumstance. Rigid bronchoscopy has also proven to be the most accurate in evaluating length of stenosis in the subglottic larynx. This is particularly important because specific surgical approaches are used in patients with subglottic tracheal stenosis to avoid recurrent laryngeal nerve injury. This higher accuracy is likely explained by the possibility of performing an accurate evaluation even in the patient with critical stenosis (72). Carretta et al. found that when used to evaluate the subglottic larynx, the sensitivity, specificity, and accuracy of RB were 67%, 100%, and 92% respectively (72).
Transbronchial lung biopsies of pulmonary lesions or diffuse parenchymal lung disease can often be obtained via flexible bronchoscopy. Rigid bronchoscopy may be employed when biopsy specimens obtained by the tools passed through a flexible scope are not sufficient or when a large specimen is anticipated to be needed (73-75). Specimens may be crushed when being removed through the fiber-optic scope making their histological examination more challenging (75). Casoni et al. found that the diagnostic yield of transbronchial lung biopsies was significantly higher (78% vs. 65%) when using jumbo forceps via RB compared to biopsies obtained using a smaller flexible biopsy forceps (76). Others feel that RB is more widely available and cheaper when compared to more sophisticated fiber-optic techniques utilized for transbronchial needle biopsy, and suggest that RB maintains better control of the airway, have shorter procedure times, and faster control of bleeding (77).
Conclusions
Airway endoscopy is a useful tool for evaluation of airway disease and an essential skill for all physicians who manage bronchopulmonary diseases. Flexible and rigid bronchoscopy are widely available for use. EBUS and ENB have increased the diagnostic accuracy of endoscopic biopsy of mediastinal and PPL. As the focus of treatment of thoracic malignancies shift toward personalized medicine, pulmonologist and surgeons will be asked to do more endoscopic biopsies for molecular tissue testing. Given this trend, the increase in diagnostic yield, and minimal risk associated with these procedures, airway endoscopy will remain critical in the evaluation of bronchopulmonary diseases and provide safe and effective approaches to tissue sampling.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Panchabhai TS, Mehta AC. Historical perspectives of bronchoscopy. Connecting the dots. Ann Am Thorac Soc 2015;12:631-41. [Crossref] [PubMed]
- Kollofrath O. Entfernung eines Knochenstücks aus dem rechten Bronchus auf natürlichem Wege und unter Anwendung der directen Laryngoscopie. MMW 1897;38:1038-9.
- Jackson C. Tracheo-bronchoscopy, esophagoscopy and gastroscopy. St. Louis, MO: The Laryngoscope Company, 1907. Available online: https://archive.org/stream/tracheobronchosc00jackuoft/tracheobronchosc00jackuoft_djvu.txt
- Walters DM, Wood DE. Operative endoscopy of the airway. J Thorac Dis 2016;8:S130-9. [PubMed]
- McRae K. Anesthesia for airway surgery. Anesthesiol Clin North America 2001;19:497-541. vi. [Crossref] [PubMed]
- Goudra BG, Singh PM, Borle A, et al. Anesthesia for Advanced Bronchoscopic Procedures: State-of-the-Art Review. Lung 2015;193:453-65. [Crossref] [PubMed]
- Merritt R, Cannon C, Kulkarni V, et al. Thoracic Surgery. In: Jaffe R, Schmiesing C, Golianu B. editors. Anesthesiologist's Manual of Surgical Procedures. Fifth ed. Philadelphia, PA: Wolters Kluwer Health, 2014:277-344.
- Pathak V, Welsby I, Mahmood K, et al. Ventilation and anesthetic approaches for rigid bronchoscopy. Ann Am Thorac Soc 2014;11:628-34. [Crossref] [PubMed]
- Nicastri DG, Weiser TS. Rigid Bronchoscopy: Indications and Techniques. Oper Tech Thorac Cardiovasc Surg 2012;17:44-51. [Crossref]
- Du Rand IA, Blaikley J, Booton R, et al. Summary of the British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults. Thorax 2013;68:786-7. [Crossref] [PubMed]
- Facciolongo N, Patelli M, Gasparini S, et al. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis 2009;71:8-14. [PubMed]
- Pue CA, Pacht ER. Complications of fiberoptic bronchoscopy at a university hospital. Chest 1995;107:430-2. [Crossref] [PubMed]
- Prokakis C, Koletsis EN, Dedeilias P, et al. Airway trauma: a review on epidemiology, mechanisms of injury, diagnosis and treatment. J Cardiothorac Surg 2014;9:117. [Crossref] [PubMed]
- Cassada DC, Munyikwa MP, Moniz MP, et al. Acute injuries of the trachea and major bronchi: importance of early diagnosis. Ann Thorac Surg 2000;69:1563-7. [Crossref] [PubMed]
- Ching JA, Ching YH, Shivers SC, et al. An Analysis of Inhalation Injury Diagnostic Methods and Patient Outcomes. J Burn Care Res 2016;37:e27-32. [Crossref] [PubMed]
- Tenenbaum T, Kähler G, Janke C, et al. Management of Foreign Body Removal in Children by Flexible Bronchoscopy. J Bronchology Interv Pulmonol 2017;24:21-8. [Crossref] [PubMed]
- Zhang L, Yin Y, Zhang J, et al. Removal of foreign bodies in children's airways using flexible bronchoscopic CO2 cryotherapy. Pediatr Pulmonol 2016;51:943-9. [Crossref] [PubMed]
- McLean AN, Semple PA, Franklin DH, et al. The Scottish multi-centre prospective study of bronchoscopy for bronchial carcinoma and suggested audit standards. Respir Med 1998;92:1110-5. [Crossref] [PubMed]
- Tenda ED, Yakub A, Pitoyo CW, et al. Combination of bronchoscopic cryoextraction and argon plasma coagulation in treatment of total central airway obstruction caused by giant blood clot formation in massive airway bleeding. Respir Med Case Rep 2016;19:9-11. [PubMed]
- Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7. [Crossref] [PubMed]
- Dhooria S, Sehgal IS, Aggarwal AN, et al. Diagnostic Yield and Safety of Cryoprobe Transbronchial Lung Biopsy in Diffuse Parenchymal Lung Diseases: Systematic Review and Meta-Analysis. Respir Care 2016;61:700-12. [Crossref] [PubMed]
- Yang H, Zhang Y, Wang KP, et al. Transbronchial needle aspiration: development history, current status and future perspective. J Thorac Dis 2015;7:S279-86. [PubMed]
- Yasufuku K, Nakajima T, Chiyo M, et al. Endobronchial ultrasonography: current status and future directions. J Thorac Oncol 2007;2:970-9. [Crossref] [PubMed]
- Chandra S, Nehra M, Agarwal D, et al. Diagnostic accuracy of endobronchial ultrasound-guided transbronchial needle biopsy in mediastinal lymphadenopathy: a systematic review and meta-analysis. Respir Care 2012;57:384-91. [PubMed]
- Nakajima T, Yasufuku K, Fujiwara T, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrapulmonary lesions. J Thorac Oncol 2008;3:985-8. [Crossref] [PubMed]
- Navani N, Molyneaux PL, Breen RA, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: a multicentre study. Thorax 2011;66:889-93. [Crossref] [PubMed]
- Huang CT, Ruan SY, Liao WY, et al. Risk factors of pneumothorax after endobronchial ultrasound-guided transbronchial biopsy for peripheral lung lesions. PLoS One 2012;7:e49125. [Crossref] [PubMed]
- Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013;143:1044-53. [Crossref] [PubMed]
- Ost D, Shah R, Anasco E, et al. A randomized trial of CT fluoroscopic-guided bronchoscopy vs conventional bronchoscopy in patients with suspected lung cancer. Chest 2008;134:507-13. [Crossref] [PubMed]
- Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015;10:331-7. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Paone G, Nicastri E, Lucantoni G, et al. Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions. Chest 2005;128:3551-7. [Crossref] [PubMed]
- Durakovic A, Andersen H, Christiansen A, et al. Retrospective analysis of radial EBUS outcome for the diagnosis of peripheral pulmonary lesion: sensitivity and complications. Eur Clin Respir J 2015;2:28947. [Crossref] [PubMed]
- Georgiou HD, Taverner J, Irving LB, et al. Safety and Efficacy of Radial EBUS for the Investigation of Peripheral Pulmonary Lesions in Patients With Advanced COPD. J Bronchology Interv Pulmonol 2016;23:192-8. [Crossref] [PubMed]
- Okachi S, Imai N, Imaizumi K, et al. Factors Affecting the Diagnostic Yield of Transbronchial Biopsy Using Endobronchial Ultrasonography with a Guide Sheath in Peripheral Lung Cancer. Intern Med 2016;55:1705-12. [Crossref] [PubMed]
- Chen CH, Cheng WC, Wu BR, et al. Improved diagnostic yield of bronchoscopy in peripheral pulmonary lesions: combination of radial probe endobronchial ultrasound and rapid on-site evaluation. J Thorac Dis 2015;7:S418-25. [PubMed]
- Dhooria S, Aggarwal AN, Gupta D, et al. Utility and Safety of Endoscopic Ultrasound With Bronchoscope-Guided Fine-Needle Aspiration in Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis. Respir Care 2015;60:1040-50. [Crossref] [PubMed]
- Erer OF, Erol S, Anar C, et al. Diagnostic yield of EBUS-TBNA for lymphoma and review of the literature. Available online: http://www.eusjournal.com/preprintarticle.asp?id=180762
- Kennedy MP, Jimenez CA, Bruzzi JF, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax 2008;63:360-5. [Crossref] [PubMed]
- Steinfort DP, Conron M, Tsui A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol 2010;5:804-9. [Crossref] [PubMed]
- Marshall CB, Jacob B, Patel S, et al. The utility of endobronchial ultrasound-guided transbronchial needle aspiration biopsy in the diagnosis of mediastinal lymphoproliferative disorders. Cancer Cytopathol 2011;119:118-26. [Crossref] [PubMed]
- Iqbal S, DePew ZS, Kurtin PJ, et al. Endobronchial ultrasound and lymphoproliferative disorders: a retrospective study. Ann Thorac Surg 2012;94:1830-4. [Crossref] [PubMed]
- Moonim MT, Breen R, Fields PA, et al. Diagnosis and subtyping of de novo and relapsed mediastinal lymphomas by endobronchial ultrasound needle aspiration. Am J Respir Crit Care Med 2013;188:1216-23. [Crossref] [PubMed]
- Ko HM, da Cunha Santos G, et al. Diagnosis and subclassification of lymphomas and non-neoplastic lesions involving mediastinal lymph nodes using endobronchial ultrasound-guided transbronchial needle aspiration. Diagn Cytopathol 2013;41:1023-30. [Crossref] [PubMed]
- Senturk A, Babaoglu E, Kilic H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Asian Pac J Cancer Prev 2014;15:4169-73. [Crossref] [PubMed]
- Dziedzic DA, Peryt A, Orlowski T. The role of EBUS-TBNA and standard bronchoscopic modalities in the diagnosis of sarcoidosis. Clin Respir J 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Agarwal R, Srinivasan A, Aggarwal AN, et al. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir Med 2012;106:883-92. [Crossref] [PubMed]
- Ye W, Zhang R, Xu X, et al. Diagnostic Efficacy and Safety of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Intrathoracic Tuberculosis: A Meta-analysis. J Ultrasound Med 2015;34:1645-50. [Crossref] [PubMed]
- Herth F, Ernst A, Schulz M, et al. Endobronchial ultrasound reliably differentiates between airway infiltration and compression by tumor. Chest 2003;123:458-62. [Crossref] [PubMed]
- Leong S, Ju H, Marshall H, et al. Electromagnetic navigation bronchoscopy: A descriptive analysis. J Thorac Dis 2012;4:173-85. [PubMed]
- Superdimension™ navigation system features. Medtronic. Available online: http://superdimension.com/innovations/superdimension-system/features/
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Hagmeyer L, Priegnitz C, Kocher M, et al. Fiducial marker placement via conventional or electromagnetic navigation bronchoscopy (ENB): an interdisciplinary approach to the curative management of lung cancer. Clin Respir J 2016;10:291-7. [Crossref] [PubMed]
- Dale CR, Madtes DK, Fan VS, et al. Navigational bronchoscopy with biopsy versus computed tomography-guided biopsy for the diagnosis of a solitary pulmonary nodule: a cost-consequences analysis. J Bronchology Interv Pulmonol 2012;19:294-303. [Crossref] [PubMed]
- Port J, Harrison S. Electromagnetic navigational bronchoscopy. Semin Intervent Radiol 2013;30:128-32. [Crossref] [PubMed]
- Zhang W, Chen S, Dong X, et al. Meta-analysis of the diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules. J Thorac Dis 2015;7:799-809. [PubMed]
- Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration 2014;87:165-76. [Crossref] [PubMed]
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a Bronchus sign on CT imaging: results from a prospective study. Chest 2010;138:1316-21. [Crossref] [PubMed]
- Diken ÖE, Karnak D, Çiledağ A, et al. Electromagnetic navigation-guided TBNA vs conventional TBNA in the diagnosis of mediastinal lymphadenopathy. Clin Respir J 2015;9:214-20. [Crossref] [PubMed]
- Karnak D, Ciledağ A, Ceyhan K, et al. Rapid on-site evaluation and low registration error enhance the success of electromagnetic navigation bronchoscopy. Ann Thorac Med 2013;8:28-32. [Crossref] [PubMed]
- Petrella F, Borri A, Casiraghi M, et al. Operative rigid bronchoscopy: indications, basic techniques and results. Multimed Man Cardiothorac Surg 2014;2014. pii: mmu006.
- Liberman M. Bronchoscopic evaluation of the trachea and dilation of the trachea. Semin Thorac Cardiovasc Surg 2009;21:255-62. [Crossref] [PubMed]
- Bronchoscopy Rigid. Chest 2003;123:1695-6. [Crossref]
- Prinja N, Manoukian JJ. Neonatal/infant rigid bronchoscopy. J Otolaryngol 1998;27:31-6. [PubMed]
- Cavel O, Bergeron M, Garel L, et al. Questioning the legitimacy of rigid bronchoscopy as a tool for establishing the diagnosis of a bronchial foreign body. Int J Pediatr Otorhinolaryngol 2012;76:194-201. [Crossref] [PubMed]
- Righini CA, Morel N, Karkas A, et al. What is the diagnostic value of flexible bronchoscopy in the initial investigation of children with suspected foreign body aspiration? Int J Pediatr Otorhinolaryngol 2007;71:1383-90. [Crossref] [PubMed]
- Martinot A, Closset M, Marquette CH, et al. Indications for flexible versus rigid bronchoscopy in children with suspected foreign-body aspiration. Am J Respir Crit Care Med 1997;155:1676-9. [Crossref] [PubMed]
- Kadmon G, Stern Y, Bron-Harlev E, et al. Computerized scoring system for the diagnosis of foreign body aspiration in children. Ann Otol Rhinol Laryngol 2008;117:839-43. [Crossref] [PubMed]
- Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980;35:901-4. [Crossref] [PubMed]
- Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010;80:38-58. [Crossref] [PubMed]
- Wedzicha JA, Pearson MC. Management of massive haemoptysis. Respir Med 1990;84:9-12. [Crossref] [PubMed]
- Carretta A, Melloni G, Ciriaco P, et al. Preoperative assessment in patients with postintubation tracheal stenosis: Rigid and flexible bronchoscopy versus spiral CT scan with multiplanar reconstructions. Surg Endosc 2006;20:905-8. [Crossref] [PubMed]
- Wilsher ML, Gurley AM. Transtracheal aspiration using rigid bronchoscopy and a rigid needle for investigating mediastinal masses. Thorax 1996;51:197-9. [Crossref] [PubMed]
- Wang KP, Britt EJ, Haponik EF, et al. Rigid transbronchial needle aspiration biopsy for histological specimens. Ann Otol Rhinol Laryngol 1985;94:382-5. [PubMed]
- Rudd RM, Gellert AR, Boldy DA, et al. Bronchoscopic and percutaneous aspiration biopsy in the diagnosis of bronchial carcinoma cell type. Thorax 1982;37:462-5. [Crossref] [PubMed]
- Casoni GL, Gurioli C, Chhajed PN, et al. The value of transbronchial lung biopsy using jumbo forceps via rigid bronchoscope in diffuse lung disease. Monaldi Arch Chest Dis 2008;69:59-64. [PubMed]
- Galluccio G, Palazzolo M, Battistoni P, et al. Role of Transbronchial Needle Core Biopsy in the Diagnosis of Mediastinal Diseases: Experience With an Innovative Endoscopic Technique Using a Rigid Tru-Cut Needle. J Bronchology Interv Pulmonol 2016. [Epub ahead of print]. [Crossref] [PubMed]


