Five years of keeping a watch on the left atrial appendage—how has the WATCHMAN fared?
Atrial fibrillation (AF) & stroke prevention
AF is the most common cardiac arrhythmia in the United States, affecting approximately 6–7 million individuals nationally, with a projected increase in prevalence to nearly 16 million patients by the year 2050 (1,2). Among the most effective cardiovascular therapies has been systemic anticoagulation with vitamin K antagonists (VKA) such as warfarin, which has been shown to reduce stroke risk in non-valvular AF by 64%, with an absolute risk reduction of 2.7% per year in patients with no history of stroke or TIA (3). Current AF management guidelines from the American College of Cardiology (ACC) and American Heart Association (AHA) provide a Class I recommendation for systemic anticoagulation with either warfarin (Level of Evidence, A) or one of a number of non-VKA oral anticoagulants (NOACs), including dabigatran, rivaroxaban, and apixaban (Level of Evidence, B) in those patients with a prior history of stroke or transient ischemic attack (TIA), as well as those with a CHA2DS2VASc score of ≥2 (4-7).
Despite the widespread availability of these therapies, there remain significant barriers to providing adequate stroke prophylaxis for many patients with AF (8). In a systematic review of studies examining current treatment practices for stroke prevention in AF, Ogilvie et al. found that in over two-thirds of studies of AF patients with prior stroke or TIA, anticoagulation treatment was prescribed in less than 60% of eligible patients (9). This concerning trend stems from a variety of factors such as perceived contraindication to anticoagulation or low stroke risk (10-13), older age and frailty (14,15), AF classification (15,16), sex (14), narrow therapeutic window (12), significant drug-drug and drug-diet interactions, and patient compliance (12,13). Moreover, even in recent large, randomized control trial settings, the time in the therapeutic range for warfarin has been measured between 55–66%, and 20–27% of patients ultimately discontinued their systemic anticoagulation therapy over a follow-up of approximately 2 years (4,5,7).
The “most lethal” appendage: site-directed therapy
These limitations in effective stroke prevention for patients with AF have prompted a search for alternative solutions. The left atrial appendage (LAA) has long been thought to serve as the major nidus for AF-related cardiac thromboemboli and has been implicated in over 90% of cases of non-valvular AF (17). Rooted in this principle, a number of therapies have emerged for mechanical closure of the LAA (LAAC), including surgical ligation and clipping, as well as percutaneous techniques featuring endocardial and epicardial approaches to the LAA (18-21). Consensus statements from both the American College of Cardiology (ACC)/Heart Rhythm Society (HRS)/Society for Cardiovascular Angiography and Interventions (SCAI) (22), as well as the European Heart Rhythm Association (EHRA)/European Association of Percutaneous Cardiovascular Interventions (EAPCI) (23), now provide some guidance regarding consideration of LAAC therapy for stroke prevention in AF, in addition to a set of institutional and operator requirements for a successful LAAC program (24).
Evaluation of the WATCHMANTM device in clinical trials
The WATCHMAN device (Boston Scientific Corp., Marlborough, MA, USA) represents the first Food and Drug Administration (FDA)-approved percutaneous LAAC device indicated for reducing the risk of thromboembolism from the LAA in patients with non-valvular AF who: (I) are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2VASc scores and are recommended for anticoagulation therapy; (II) are deemed by their physicians to be suitable for warfarin; and (III) have an appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and efficacy of the device compared to warfarin. This approval was granted by the FDA in March 2015 following a prolonged pre-market approval pathway process featuring two randomized control trials (PROTECT AF and PREVAIL) to study its non-inferiority to warfarin and two prospective registries (CAP and CAP2) to monitor safety and efficacy of the device over time (25-28). In PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation), despite achieving non-inferiority versus warfarin in the combined efficacy endpoint of ischemic or hemorrhagic stroke, systemic thromboembolism, and cardiovascular or unexplained death (3 vs. 4.9 events per 100 patient-years, RR 0.62; 95% CI: 0.35–1.25), the WATCHMAN device raised concerns with a higher rate of primary safety events (7.4 vs. 4.4 events per 100 patient-years, RR 1.69; 95% CI: 1.01–3.19) mainly related to periprocedural complications such as pericardial effusion (4.8%), major bleeding (3.5%), and procedure-related stroke (1.1%) (20).
Further study of the device in the PREVAIL (Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device In Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy) trial failed to demonstrate achievement of a pre-specified, composite efficacy endpoint of stroke, systemic embolism, and cardiovascular/unexplained death (the same composite endpoint used in PROTECT AF; 0.064 in device group vs. 0.063 in warfarin group, RR 1.07; 95% CI: 0.57–1.89) (26). The device, however, did meet its second co-primary efficacy endpoint (referred to as the “late ischemic efficacy” endpoint) defined as occurrence of ischemic stroke and systemic embolism beyond 7 days post-randomization and over the follow-up period of 18 months, effectively excluding peri-procedural events given the unique nature of comparison between a device and a drug. The WATCHMAN device similarly met its safety co-primary endpoint in PREVAIL. In a subsequent meeting of the FDA Circulatory Systems Advisory Panel in October 2014, newly available data including eight ischemic strokes in the WATCHMAN group resulted in the reassessment that the device did not meet its second pre-specified co-primary endpoint and failed to demonstrate non-inferiority to warfarin in PREVAIL (22,29). It has been noted, however, that the rate of ischemic strokes in the warfarin control group of PREVAIL was less than half that observed in three recent major trials of NOACs (4,5,7,26), fueling controversy surrounding interpretation of data from yet another WATCHMAN randomized control trial. Ultimately FDA approval was granted in March 2015 for the nuanced indication noted previously. Incorporation of all available trial and registry data in a patient-level meta-analysis has since supported a statistically significant reduction in hemorrhagic stroke, non-procedure-related bleeding, and cardiovascular death with the WATCHMAN (25). However, the PREVAIL findings and FDA concerns surrounding overall efficacy as compared to warfarin, particularly in the case of ischemic stroke, have emphasized the need for rigorous post-marketing surveillance and long-term follow-up of patients receiving the WATCHMAN device.
Extending the follow-up on WATCHMAN
The longest reported follow-up with WATCHMAN to date has been the 4-year PROTECT AF experience reported by Reddy et al. with a mean follow-up duration of 3.8±1.7 years (28). For the composite efficacy endpoint of stroke, systemic embolism, and cardiovascular death, the WATCHMAN group had 39 events among 463 patients (8.4%) vs. 34 events in 244 patients (13.9%) in the warfarin group (event rate, 2.3 vs. 3.8 per 100 patient-years; RR 0.60; 95% CI: 0.41–1.05), meeting the trial’s non-inferiority criteria and demonstrating significant reductions in cardiovascular and all-cause mortality in secondary analyses. The beneficial outcome demonstrated with the WATCHMAN device was attributed largely to reductions in hemorrhagic stroke and cardiovascular death. Ischemic stroke rates in the two groups were not significantly different, though again this result must be interpreted in the context of the subsequent PREVAIL trial, which though it enrolled fewer patients [device group, 463 (PROTECT AF) vs. 269 (PREVAIL)], did not demonstrate the non-inferiority of the WATCHMAN as assessed by its two co-primary efficacy endpoints.
With respect to safety, the four-year PROTECT AF data demonstrated a time-dependent distribution of safety events with the WATCHMAN device, consisting of peri-procedural (up to 7 days) serious pericardial effusion in 22/463 (4.8%), procedure-related ischemic stroke in 5 (1.1%), and device embolization in 3 (0.6%). The number of events beyond 7 days post-implantation was considerably less, with major bleeding in 19 (4.1%) compared with 18 (7.4%) in the warfarin group, procedure-related ischemic stroke in 1 (0.2%), and hemorrhagic stroke in 3 (0.6%) compared to 9 (3.7%) in the warfarin group (28). In an intention-to-treat analysis combining all safety events, there was no significant difference between the two groups. In PREVAIL, primary safety events occurred in 6/269 (2.2%) WATCHMAN patients over 18 months follow-up. Due to the unique comparison of a device versus a medication, safety events were not reported for the warfarin group separately, using instead a Bayesian model incorporating data from the prior PROTECT AF study and CAP Registry to compute a performance goal of 2.67% for the WATCHMAN group in PREVAIL. The “early safety” primary endpoint was a composite of all-cause death, ischemic stroke, systemic embolism, or device-/procedure-related events requiring open cardiovascular surgery or major endovascular intervention between randomization and 7 days after the procedure or during the index hospitalization. It was met with the upper bound of the one-sided 95% credible interval computed at 2.652% for the WATCHMAN group.
Recently, Wiebe et al. described a relatively large single-center experience of 102 AF patients treated with the WATCHMAN device with up to 5 years follow-up (30). Patients had mean CHA2DS2VASc and HAS-BLED scores of 4.3±1.7 and 2.9±1.2, respectively. Procedural success was 96.1% (98/102), exceeding the 91% reported in PROTECT AF and in line with previously published trial and registry data from PREVAIL, CAP (Continued Access Protocol) registry, and the ASAP (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology) registry (26,27,31).
During a mean follow-up of 3.0±1.6 years, second in duration only to the PROTECT AF four-year follow-up experience (28), two patients (0.7 per 100 patient-years) had ischemic strokes, as compared to 1.4 per 100 patient-years in PROTECT AF and less than the study group’s CHA2DS2VASc-predicted stroke risk of 4–6.7% annually. Two patients had TIAs—one at one month and the other beyond 12 months post-implantation. Three patients suffered intracranial bleeding events for a rate of 1.1 per 100 patient-years, which exceeded the rate in PROTECT AF by nearly six-fold (0.2 per 100 patient-years), though it is not reported what percentage of patients continued warfarin long-term, which may explain some portion of the bleeding events. Severe bleeding events occurred in six patients (6.3%) compared to 4.8% in PROTECT AF (28). Freedom from all-cause mortality at 60 months was just less than 82.5%, while this figure was approximately 86% in the PROTECT AF device group at the same point in time.
Device-related thrombi and the anticoagulation conundrum
Importantly, in the study by Wiebe et al., a significant portion of patients (41/98) were exclusively administered dual antiplatelet therapy (DAPT) post-implantation, while the remaining 57 (58.2%) received the usual VKA for 45 days followed by 6 months of DAPT (30). This was a notable deviation from the protocol utilized in the WATCHMAN trials to treat patients with VKA for the first 45 days post-implantation, followed by DAPT for 6 months. The authors reported that 25 patients were not eligible for anticoagulation. Despite this difference in management, there were device-related thrombi (DRT) in only two cases (4.9%), in addition to one ischemic event, in the DAPT group (Figure 1). It is difficult to draw conclusions given the overall low event rate, however in the ASAP Study of 150 warfarin ineligible patients, there were a total of 6 (4%) device-related thrombi and one thought to be implicated in an ischemic stroke (31). This trend of foregoing post-procedural anticoagulation with VKA is more representative of European practice patterns given guideline recommendations to consider LAAC in patients in whom anticoagulation is contraindicated, though it is important to note there are no randomized control trial data studying LAAC in this scenario, as these patients were excluded from the WATCHMAN trials. Nevertheless, it is a key patient population which is in need of a safe and effective alternative for stroke prevention.

In a post hoc analysis of the PROTECT AF study population, Main et al. found that in 35/485 (7.2%) patients receiving a WATCHMAN device who were suspected by the site investigator and/or the echocardiography core laboratory to have a DRT, 27 were ultimately adjudicated by a panel of three echocardiographers to have had a DRT in one of their post-procedure studies (32). In addition to illustrating the challenge of making the diagnosis, 19 of 33 (56.7%) with an available TEE study had a thrombus detected at the 6-month post-implantation follow-up, while 12/27 (44.4%) with an available TEE study at 12 months post-implantation had a DRT. The primary composite efficacy endpoint of PROTECT AF (stroke, systemic thromboembolism, cardiovascular/unexplained death) was detected in patients with DRT at a rate of 3.4 per 100 patient-years, intermediate in frequency between the device in and warfarin groups in the PROTECT AF study (32).
These findings highlight one of the major challenges and areas for further investigation with the WATCHMAN device vis-à-vis peri-procedural and post-procedural management of LAAC patients. The significance of DRTs and their prevention remain poorly understood. Some considerations include: (I) the possibility that the duration of current anticoagulation/antiplatelet protocols is inadequate in some patients for proper endothelialization over the LAA ostium; (II) the combination of DAPT is insufficient in protecting against thrombus formation; or (III) there are device- and/or patient-related factors which predispose some individuals to thrombus formation (e.g., threaded insert of the device). Furthermore, despite the arbitrary yet commonly employed de-escalation protocol—from aspirin and VKA to DAPT at 45 days and subsequently to aspirin monotherapy after six additional months—a substantial number of patients are not able to be liberated from VKA therapy at 12-month follow-up. This number approaches 7% in the PROTECT AF study population of carefully selected patients treated by experienced operators and is likely to be greater in a “real world” population (28).
Plugging the dike and patient-occluder mismatch
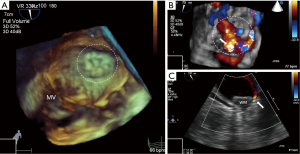
Contributing to the issue is the fact that our understanding of the significance and future ramifications of peri-device leaks remains incomplete, particularly in those cases where the leak exceeds 5 millimeters (Figure 2). In a sub-study of the PROTECT AF device group limited by low power and post hoc analysis, it was noted that 32% of patients had some residual peri-device flow at 12-month follow-up, but that neither the severity of the leak nor the administration of VKA therapy seemed to correlate with the primary combined efficacy endpoint of that trial (33). Closely related to the issue of leaks are the challenges posed by the anatomical variation of the LAA and the elliptical morphology of the LAA ostium (34,35). With the advent of new technologies for closure of the LAA, it is hoped that many of these obstacles can be overcome.
Occluder devices featuring a “disc and lobe” configuration such as the AMPLATZER™ Amulet™ (St. Jude Medical, St. Paul, MN, USA), which carries the Conformité Européenne (CE) mark and is widely used in Europe, show promise in offering greater versatility for a variety of LAA morphologies (36,37), though in a small canine study some concern was raised regarding potential interference of the disc with surrounding structures, including the left superior pulmonary vein and the mitral valve apparatus (38). Another CE-marked and also FDA-approved option, the LARIAT® Suture Delivery Device (SentreHEART, Redwood City, CA, USA), features an entirely unique, hybrid (endocardial and epicardial) approach to closing the LAA (21,39). The latter two devices are each currently the subject of a randomized control trial (ClinicalTrials.gov Identifiers: NCT02879448, NCT02513797) (40). Additional LAAC devices, including the WaveCrest (Coherex Medical, Salt Lake City, UT, USA) device, the Occlutech LAA Occluder (Occlutech International AB, Helsingborg, Sweden) (41), and the LAmbre (Lifetech Scientific, Shenzhen, China) device (42), are in various stages of development.
Conclusions and future considerations for LAA-directed therapies
The development and approval of the WATCHMAN device heralds a new era in “appendage-ology” in which it is conceivable that emerging therapies will equip LAAC specialists with an armamentarium capable of providing the right LAAC therapy for the right patient (Figure 3). The WATCHMAN has laid the groundwork for this exciting prospect and has confirmed the significance of the LAA in AF-related stroke mechanisms. Despite the need for further study to understand its proper role in the overall approach to stroke prophylaxis, particularly with respect to preventing ischemic stroke, it has also shown a significant reduction in the rate of hemorrhagic stroke, non-procedure-related bleeding, and cardiovascular death. Furthermore, the importance of the LAA in arrhythmia propagation and neurohormonal regulation has been established (43-45), and it would be remiss to avoid their consideration in a comprehensive approach to appendage closure. These aspects of LAAC therapy warrant further investigation, as they may shed additional light on the significance of this most lethal appendage, as well as how its successful closure may confer pleiotropic effects to AF patients.

Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370-5. [Crossref] [PubMed]
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119-25. [Crossref] [PubMed]
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67. [Crossref] [PubMed]
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [Crossref] [PubMed]
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. [Crossref] [PubMed]
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1-76. [Crossref] [PubMed]
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. [Crossref] [PubMed]
- Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med 2000;160:41-6. [Crossref] [PubMed]
- Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 2010;123:638-645.e4. [Crossref] [PubMed]
- Gattellari M, Worthington J, Zwar N, et al. Barriers to the use of anticoagulation for nonvalvular atrial fibrillation: a representative survey of Australian family physicians. Stroke 2008;39:227-30. [Crossref] [PubMed]
- Levine MN, Raskob G, Landefeld S, et al. Hemorrhagic complications of anticoagulant treatment. Chest 2001;119:108S-121S. [Crossref] [PubMed]
- O'Brien EC, Holmes DN, Ansell JE, et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J 2014;167:601-609.e1. [Crossref] [PubMed]
- Reynolds MR, Shah J, Essebag V, et al. Patterns and predictors of warfarin use in patients with new-onset atrial fibrillation from the FRACTAL Registry. Am J Cardiol 2006;97:538-43. [Crossref] [PubMed]
- Lewis WR, Fonarow GC, LaBresh KA, et al. Differential use of warfarin for secondary stroke prevention in patients with various types of atrial fibrillation. Am J Cardiol 2009;103:227-31. [Crossref] [PubMed]
- Waldo AL, Becker RC, Tapson VF, et al. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol 2005;46:1729-36. [Crossref] [PubMed]
- Glazer NL, Dublin S, Smith NL, et al. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med 2007;167:246-52. [Crossref] [PubMed]
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755-9. [Crossref] [PubMed]
- Emmert MY, Puippe G, Baumüller S, et al. Safe, effective and durable epicardial left atrial appendage clip occlusion in patients with atrial fibrillation undergoing cardiac surgery: first long-term results from a prospective device trial. Eur J Cardiothorac Surg 2014;45:126-31. [Crossref] [PubMed]
- Healey JS, Crystal E, Lamy A, et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 2005;150:288-93. [Crossref] [PubMed]
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534-42. [Crossref] [PubMed]
- Lakkireddy D, Afzal MR, Lee RJ, et al. Short and long-term outcomes of percutaneous left atrial appendage suture ligation: Results from a US multicenter evaluation. Heart Rhythm 2016;13:1030-6. [Crossref] [PubMed]
- Masoudi FA, Calkins H, Kavinsky CJ, et al. 2015 ACC/HRS/SCAI Left Atrial Appendage Occlusion Device Societal Overview. J Am Coll Cardiol 2015;66:1497-513. [Crossref] [PubMed]
- Meier B, Blaauw Y, Khattab AA, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. Europace 2014;16:1397-416. [Crossref] [PubMed]
- Kavinsky CJ, Kusumoto FM, Bavry AA, et al. SCAI/ACC/HRS institutional and operator requirements for left atrial appendage occlusion. Catheter Cardiovasc Interv 2016;87:351-62. [Crossref] [PubMed]
- Holmes DR Jr, Doshi SK, Kar S, et al. Left Atrial Appendage Closure as an Alternative to Warfarin for Stroke Prevention in Atrial Fibrillation: A Patient-Level Meta-Analysis. J Am Coll Cardiol 2015;65:2614-23. [Crossref] [PubMed]
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1-12. [Crossref] [PubMed]
- Reddy VY, Holmes D, Doshi SK, et al. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation 2011;123:417-24. [Crossref] [PubMed]
- Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014;312:1988-98. [Crossref] [PubMed]
- Transcript of the Circulatory System Devices Panel Meeting—October 8, 2014. Available online: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/CirculatorySystemDevicesPanel/UCM421983.pdf, accessed August 23, 2016.
- Wiebe J, Franke J, Lehn K, et al. Percutaneous Left Atrial Appendage Closure With the Watchman Device: Long-Term Results Up to 5 Years. JACC Cardiovasc Interv 2015;8:1915-21. [Crossref] [PubMed]
- Reddy VY, Möbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol 2013;61:2551-6. [Crossref] [PubMed]
- Main ML, Fan D, Reddy VY, et al. Assessment of Device-Related Thrombus and Associated Clinical Outcomes With the WATCHMAN Left Atrial Appendage Closure Device for Embolic Protection in Patients With Atrial Fibrillation (from the PROTECT-AF Trial). Am J Cardiol 2016;117:1127-34. [Crossref] [PubMed]
- Viles-Gonzalez JF, Kar S, Douglas P, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol 2012;59:923-9. [Crossref] [PubMed]
- Kerut EK. Anatomy of the left atrial appendage. Echocardiography 2008;25:669-73. [Crossref] [PubMed]
- Su P, McCarthy KP, Ho SY. Occluding the left atrial appendage: anatomical considerations. Heart 2008;94:1166-70. [Crossref] [PubMed]
- Tzikas A, Shakir S, Gafoor S, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention 2016;11:1170-9. [Crossref] [PubMed]
- Tzikas A, Bergmann MW. Left atrial appendage closure: patient, device and post-procedure drug selection. EuroIntervention 2016;12 Suppl X:X48-X54.
- Kar S, Hou D, Jones R, et al. Impact of Watchman and Amplatzer devices on left atrial appendage adjacent structures and healing response in a canine model. JACC Cardiovasc Interv 2014;7:801-9. [Crossref] [PubMed]
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62:108-18. [Crossref] [PubMed]
- Lee RJ, Lakkireddy D, Mittal S, et al. Percutaneous alternative to the Maze procedure for the treatment of persistent or long-standing persistent atrial fibrillation (aMAZE trial): Rationale and design. Am Heart J 2015;170:1184-94. [Crossref] [PubMed]
- Kim JS, Lee SG, Bong SK, et al. Preclinical assessment of a modified Occlutech left atrial appendage closure device in a canine model. Int J Cardiol 2016;221:413-8. [Crossref] [PubMed]
- Lam YY. A new left atrial appendage occluder (Lifetech LAmbre Device) for stroke prevention in atrial fibrillation. Cardiovasc Revasc Med 2013;14:134-6. [Crossref] [PubMed]
- Afzal MR, Kanmanthareddy A, Earnest M, et al. Impact of left atrial appendage exclusion using an epicardial ligation system (LARIAT) on atrial fibrillation burden in patients with cardiac implantable electronic devices. Heart Rhythm 2015;12:52-9. [Crossref] [PubMed]
- Han FT, Bartus K, Lakkireddy D, et al. The effects of LAA ligation on LAA electrical activity. Heart Rhythm 2014;11:864-70. [Crossref] [PubMed]
- Maybrook R, Pillarisetti J, Yarlagadda V, et al. Electrolyte and hemodynamic changes following percutaneous left atrial appendage ligation with the LARIAT device. J Interv Card Electrophysiol 2015;43:245-51. [Crossref] [PubMed]


