Percutaneous treatment of persistent chylothorax: technical challenges in a complex case
Dear Editor,
We thank Dr. Kaiser for his interest in our paper (1). We are grateful for the insightful comments about the complexity of management of chylous leak (CL) as well as the potentiality of non-operative approaches, which was initially described by his group in 1999 (2).
Lymphorrhea is a rare but potentially fatal complication that may occur anywhere in the entire lymphatic system. The chylothorax caused by damage to the thoracic duct (TD) is particularly difficult to manage, often requiring surgical repair (3).
The percutaneous transabdominal catheterization of TD it is not an easy task, despite being a safe and effective way to treat CL (4). First of all, it is necessary a good image of the cistern chili (CC) provided by a reliable lymphangiogram, a very tricky procedure per se. The ultrasound-guide intranodal lymphangiogram has been used as a better strategy than the classical painful and technically difficult pedal lymphatic cannulation (5).
However, the TD catheterization is not possible for a relevant group of patients, who are referred to minimally invasive percutaneous treatment of CL, most often because of nonvisualization of CC/TD or the impossibility to catheterize them (6).
Embolization under CT-guided imaging was proven to be effective and safe once anatomical and percutaneous landmarks are easily depicted, having some successful cases reported already (7,8). Coils and N-butyl cyanoacrylate are the preferred embolization agents (3), either followed or not by sclerosis with absolute ethanol and/or polidocanol foam.
The complex case we reported—the catheterization of TD was not possible because it had been previously clamped surgically. Several surgical approaches have been performed at foreign hospitals, and our surgical team has considered non-reasonable to repeat the same failed strategies. The only possible approach was direct percutaneous embolization through the leakage site, which was localized by contrast inserted through a pleural pigtail tube. Based on previous experience, we have acquired promising results with percutaneous sclerosis of retroperitoneal CL following lymph node dissection during robot-assisted prostatectomies and other oncological surgeries
Because of this we were encouraged to try a similar strategy in the thoracic compartment; as successfully described in our paper (Figure 1).
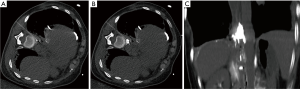
In our opinion, and as wisely pointed out by Dr. Kaiser, interventional radiologist should be familiar with the anatomy and variations of the lymphatic system, therefore, been able to perform safe and effective treatment strategies for unfortunate TD leakage patients as part of a multidisciplinary team.
Once again, we are grateful to be able to discuss our work with a pioneering group in this technique.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Garcia RG, Rocha RD, Franceschini J, et al. Computed Tomography-Guided Percutaneous Thoracic Duct Sclero-Embolization for Persistent Chylothorax. Innovations (Phila) 2016;11:291-4. [Crossref] [PubMed]
- Cope C, Salem R, Kaiser LR. Management of chylothorax by percutaneous catheterization and embolization of the thoracic duct: prospective trial. J Vasc Interv Radiol 1999;10:1248-54. [Crossref] [PubMed]
- Inoue M, Nakatsuka S, Yashiro H, et al. Lymphatic Intervention for Various Types of Lymphorrhea: Access and Treatment. Radiographics 2016;36:2199-211. [Crossref] [PubMed]
- Itkin M, Kucharczuk JC, Kwak A, et al. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg 2010;139:584-89; discussion 589-90. [Crossref] [PubMed]
- Lee EW, Shin JH, Ko HK, et al. Lymphangiography to treat postoperative lymphatic leakage: a technical review. Korean J Radiol 2014;15:724-32. [Crossref] [PubMed]
- Pamarthi V, Stecker MS, Schenker MP, et al. Thoracic duct embolization and disruption for treatment of chylous effusions: experience with 105 patients. J Vasc Interv Radiol 2014;25:1398-404. [Crossref] [PubMed]
- Itou C, Koizumi J, Myojin K, et al. A case of refractory chylous ascites after nephrectomy successfully treated with percutaneous obliteration using adhesive glue. Jpn J Radiol 2013;31:71-4. [Crossref] [PubMed]
- Kuyumcu G, Gordon R, Ott H, et al. CT-Guided Thoracic Duct Embolization. J Vasc Interv Radiol 2016;27:1753-5. [Crossref] [PubMed]


