Robotic-assisted double-sleeve lobectomy
Introduction
For the patients with central-type lung cancer, the bronchial opening and pulmonary artery can be both invaded by the tumor tissue, thus the double-sleeve lobectomy is required. This surgery is strictly challenging, which includes the bronchoplasty and pulmonary arterial angioplasty. At present, it is usually done by lateral thoracotomy or video-assisted thoracoscopic surgery (VATS). Anecdotal evidences have described the application of VATS in double-sleeve lobectomy (1,2), but sometimes there is troublesome: the angle of needle insertion for instance. As an academic thoracic surgery centre performing VATS for a dozen years, we have recently introduced the da Vinci Robotic System (Intuitive Surgical, Inc., Mountain View, California, USA) since October 2014. Here we describe a case of robotic-assisted thoracic surgery (RATS) double-sleeve lobectomy for central-type lung cancer of left upper lobe (LUL).
Case presentation
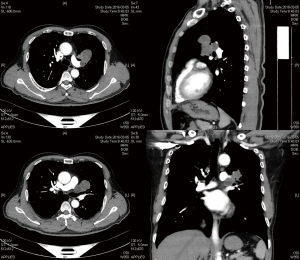
The ethics committee of Qingdao University has given the ethics approval for this manuscript. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A 57-year-old male presenting with cough and wheezing was admitted in our hospital by the diagnosis of lung cancer. The thoracic computed tomography scan revealed an occupying lesion located in the left hilum with mediastinal lymph nodes enlargement (Figure 1). The preoperative bronchoscopy and biopsy indicated carcinoma (unspecific type), which obstructed the LUL bronchial opening. Before surgery, no distant metastasis was detected. Routine laboratory studies were normal. The pulmonary function test was normal. The preoperative diagnosis was primary lung cancer, cT2bN2M0, IIIA stage (resectable).
Surgical technique
Body position
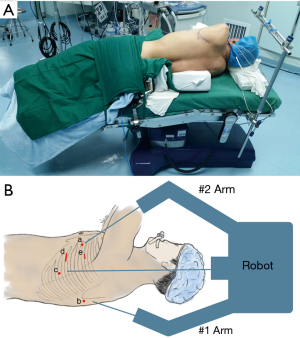
The patient is placed in a right lateral decubitus position (Figure 2A). The waist bridge is elevated in a Jackknife fashion to maximize the intercostal spaces and amplify the room for the thoracoscope.
Anesthesia
The patient is induced by general anesthesia with double-lumen endotracheal intubation.
Incisions and position of da Vinci system
The two main ports are about 1 cm which locate in the fourth intercostal space at the anterior axillary line (front port), and in the seventh intercostal space at the scapular line (back port), respectively. The camera port locates in the seventh intercostal space at the midaxillary line. The assisted incision is created in the sixth intercostal space at the anterior axillary line. After the incisions are created, the da Vinci system approaches from the head of the patient (Figure 2B). The 1# arm (right arm) is inserted from the back port, which will install the unipolar cautery hook, the needle holder and the scissors. The 2# arm (left arm) is inserted from the front port, which will install the bipolar cautery forceps and the secondary needle holder. The assistant uses the regular thoracoscopic instruments and delivers the sutures through the assisted incision.
Surgical techniques
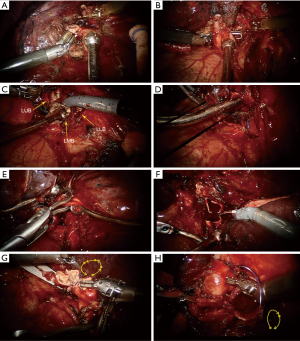
- The operation was performed on 12th March, 2016 (Figure 3). The lesion locates in the LUL around the left upper hilum, companying with lymph nodes enlargement in 5th and 10th station. Dissect the mediastinal pleura with the unipolar cautery hook. Dissect the 4L, 5, 6, 7, 8, 9, 10L, 11L station lymph nodes.
- After the lung is retracted to the back, dissociate the ligular artery and transect it by the endoscopic stapler (Figure 4A). Cut open the fissure by the endoscopic stapler. Then, the superior pulmonary vein is isolated (Figure 4B). Transect the vein by the endoscopic stapler (through the back port). Dissociate the left main bronchus, the proximal left upper bronchus and the left lower bronchus.
- Replace the hook to the scissors. Cut open both the left main bronchus and the left lower bronchus in vertical direction to the bronchial wall (Figure 4C). The cutting margins keep about one cartilage away to the opening of left upper bronchus.
- With a slight lifting of the upper bronchial stump, it is detected that the arterial branches of LUL and partial wall of the pulmonary trunk are invaded by the tumor tissue. A 1 cm incision is created in the 3rd intercostal space, through where a curved blocking clamps (Jinzhong, China) is placed to block off the proximal pulmonary trunk (Figure 4D). Careful attention should be paid to prevent the recurrent laryngeal nerve injury. A curved bulldog clamps (Aesculap, USA) is inserted via the assisted incision to block off the distal pulmonary trunk (Figure 4E).
- Check the pulmonary trunk fully blocked. At this point, anesthesiologist should slow down the infusion speed. After completing the isolation of the blocked pulmonary trunk, cut open the pulmonary trunk and remove the invaded arterial wall (Figure 4F). The LUL, which has been put into the specimen bag, is temporarily kept in the lower thoracic cavity. Then, shear the resection margins of the pulmonary artery.
- Install two needle holders on both arms. Perform the pulmonary arterial angioplasty using a 5-0 prolene suture (ETHICON 13 mm 1/2c, USA) in a short continuous style (Figure 4G). After the first 4 to 5 sutures are made, the other 4 to 5 sutures are relayed by a new prolene suture. The knots are made by the needle holders. The fashion of short continuous suture is preferred to use because that: the da Vinci system has no force feedback, so the powerful grasping force will damage the distal suture when pulling it, that would induce the 5-0 prolene easy to break. During the suturing and before the last knotting, the arterial lumen is rinsed by heparin solution regularly.
- After removing the blocking clamps, retract the lung to the front and expose the bronchial ends from the posterior hilum. The anastomosis of bronchus begins from the juncture of bronchial membranes and cartilages, using two 3-0 prolene sutures (ETHICON 24 mm 1/2c, USA, one for cartilages, the other for membranes) in a continuous suture (Figure 4H).

Outcome
No air leakage is noted with a sustained airway pressure of 25 cmH2O. Frozen sections of resection margins are negative. Intraoperative blood loss was about 150 mL, and operative time was about 240 minutes. The chest tube is placed through the camera port and removed on the postoperative day 3. The patient is discharged on postoperative day 6. The patient is followed-up for 6 months. No mortality or major morbidity presents. The finial pathologic examination is pulmonary paraganglioma with lymph nodes metastasis in 5th and 10th station. After surgery, the patient received adjuvant chemotherapy.
Discussion
RATS lobectomy has been applied in many centres of thoracic surgery all over the world. After the exploration by the early pioneers, the indication for RATS is increasingly closer to the VATS now. The feasibility of bronchoplasty in RATS has been researched for years. Ishikawa et al. investigated a male cadaver and showed that RATS bronchoplasty offered specific advantages over conventional bronchoplasty with accuracy and safety (4). Schmid et al. reported a case for hybrid VATS-RATS sleeve lobectomy in a 30-year-old female patient (5). Dylewski et al. demonstrated 200 consecutive RATS cases which included 3 sleeve lobectomies (6). Pan et al. reported a case on RATS extended sleeve lobectomy after neoadjuvant chemotherapy (7). Cerfolio reported the satisfying early outcomes of 8 patients who were scheduled for a planned RATS sleeve resection (8). However, central-type lung cancer can invade not only the bronchus but also the pulmonary artery, thus the procedure of bronchoplasty/pulmonary arterial angioplasty, also known as double-sleeve lobectomy, is needed. This procedure is strictly challenging even by conventional thoracotomy. Although this procedure in VATS has been increasingly reported in recent years, performing by RATS has been rarely reported in literatures. But as the RATS bronchoplasty has been applied maturely in several centres including ours, it is technically possible to perform it for those kinds of central-type lung cancer patients.
The blocking method of proximal pulmonary trunk is similar to VATS double-sleeve lobectomy. In He et al.’s report (2), the blocking clamps incision is made in the 2nd intercostal space on the anterior chest wall. But we change it more posterior and lower, as it is made in the 3rd intercostal space near the midaxillary line, to avoid interruption from the 2# arm. During the procedure, critical attention should be taken to prevent the robotic arm interrupting the blocking clamps. To make sure the trunk is fully blocked, we prefer using a silk twining around the trunk first. When the blocking clamps are being placed, the arterial trunk is slightly retracted by the silk to emaciate the thick pulmonary trunk. The distal pulmonary trunk is often blocked by a bulldog clamps which is as the same as that in VATS.
The pulmonary arterial angioplasty is performed before the bronchoplasty, so as to shorten the time required for pulmonary artery blockage. During the angioplasty, 5-0 prolene is chosen to make the anastomosis. This suture is fine enough to decrease the vascular intimal injury, however it is easy to break when the powerful holder pulling it. So we prefer to use a short continuous suture style which might gain some operating time but decrease the probability of accidental suture breaking. In our experience of VATS pulmonary arterial angioplasty, continuous with one 4-0 prolene can also be chosen. The bronchoplasty method is described before that we used in VATS (9). First we use 3-0 prolene to perform running suture from the anterior beginning of the pars cartilages. When the suture reaches the end of the pars cartilages, the second 3-0 prolene is knotted with the latter one and relayed to suture the pars membranes. At last these two sutures are knotted on the beginning of the first suture. This method could decompose the tension from the pars cartilages when suturing the pars membranes. When necessary, the assistant can use a rolling gauze to retract the pulmonary trunk instead of a silk suture.
To our knowledge, a rich experienced operator can complete vascular anastomosis in VATS smoothly, however, the robotic system can provide unparalleled controlling of needle insertion; furthermore, it provided a detailed and magnified 3D view, which can increase the accuracy and continuity of suturing. Nevertheless, the double-sleeve lobectomy is still challenging even by RATS. We prepared to transit to open surgery and pneumonectomy for backup plan.
There are some limits in this case. First, to our knowledge, it is a first case report of robotic double-sleeve lobectomy in literatures. More surgical experience should be gained. Second, though the patient had a short-term benefit from the robotic technique, the long-term oncological prognosis should be followed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Liu L, Mei J, Pu Q, et al. Thoracoscopic bronchovascular double sleeve lobectomy for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;46:493-5. [Crossref]
- Xu X, Huang J, Pan H, et al. Video-assisted thoracoscopic bronchoplasty/pulmonary arterial angioplasty. J Thorac Dis 2016;8:544-52. [Crossref]
- Qiu T, Zhao Y, Xuan Y, et al. Video of robotic-assisted bronchoplasty/pulmonary arterial angioplasty. Asvide 2017;4:013. Available online: http://www.asvide.com/articles/1319
- Ishikawa N, Sun YS, Nifong LW, et al. Thoracoscopic robot-assisted bronchoplasty. Surg Endosc 2006;20:1782-3. [Crossref]
- Schmid T, Augustin F, Kainz G, et al. Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg 2011;91:1961-5. [Crossref]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref]
- Pan X, Chen Y, Shi J, et al. Robotic Assisted Extended Sleeve Lobectomy After Neoadjuvant Chemotherapy. Ann Thorac Surg 2015;100:e129-31. [Crossref]
- Cerfolio RJ. Robotic sleeve lobectomy: technical details and early results. J Thorac Dis 2016;8:S223-6.
- Jiao W, Zhao Y, Wang X, et al. Video-assisted thoracoscopic left upper lobe sleeve lobectomy combined with pulmonary arterioplasty via two-port approach. J Thorac Dis 2014;6:1813-5.