Potency of parenteral antimicrobials including ceftolozane/tazobactam against nosocomial respiratory tract pathogens: considerations for empiric and directed therapy
Introduction
Nosocomial pneumonia, defined as a pulmonary infection that develops 48 hours after admission is the second most common infection in the hospital setting (1). As a result of its prevalence and the frequent debilitated nature of the infected host this disease entity is associated with high morbidity, mortality increased length of hospital stay and cost of care (1). Moreover, this infection is responsible for the majority of antibiotics prescribed in the ICU setting.
Although the epidemiology of nosocomial pneumonia has been well defined and commonly involves Staphylococcus aureus and a variety of Gram-negative pathogens such as P. aeruginosa (PSA), E. coli, and K. pneumoniae the prevalence of these pathogens as well as their susceptibility to commonly utilized antibiotics can vary widely among the global geographies of clinical care (1,2). While the combination of early recognition, diagnostics and prompt medical management have assisted in improving clinical outcomes of the infected patient, antibiotic resistant organisms present a major threat to the well-being of all patients with infection (3).
As a result of this threat, multiple initiatives have been undertaken among the pharmaceutical industry, clinicians and regulatory bodies to develop new antibiotic therapies targeted at these increasingly prevalent resistant organisms. One such effort has resulted in the recent approval of a novel β-lactam/β-lactamase inhibitor, ceftolozane/tazobactam (C/T) (ZERBAXATM), which is indicated in the US for adult patients with complicated intra-abdominal infections in combination with metronidazole and complicated urinary tract infections, including pyelonephritis (4). This cephalosporin-class agent displays potent in vitro activity against common Gram-negative pathogens such as E. coli, K. pneumoniae, P. mirabilis and PSA, including drug-resistant strains, and some extended spectrum β-lactamases (ESBL) producing Enterobacteriaceae (4,5). In the context of nosocomial infection when concerns of PSA and antibiotic resistance become increasingly prevalent, this compound has displayed high potency in the wake of non-susceptibility to agents like meropenem (MEM), piperacillin-tazobactam (TZP) and cefepime (FEP) as well as multi-drug resistance (MDR), defines as resistant to ≥3 classes of agents (6,7). As a result of the bronchopulmonary penetration of C/T, as well as it’s in vitro potency against Gram-negative pathogens including MDR PSA the compound is currently under study for ventilated nosocomial pneumonia (8,9). The purpose of this study was to compare the in vitro potency of conventional parenteral antimicrobials and C/T against nosocomial respiratory isolates from US hospitals as an aid to optimal antimicrobial(s) selection for the management of pneumonia in the hospital setting.
Methods
Consecutive, non-duplicate, Gram-negative nosocomial respiratory isolates that had been obtained from adult inpatients as part of their routine medical management were obtained from cultures received ≥48 h after hospital admission. Collection occurred over the period of July 2013 and November 2014. Routine testing methods were performed at each participating site to identify the organisms prior to shipment to the central laboratory, Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT.
Clinical Laboratory Standards Institute (CLSI) broth microdilution methods for minimum inhibitory concentration (MIC) determinations were undertaken on: C/T, FEP, ceftriaxone (CRO), ceftazidime (CAZ), ciprofloxacin (CIP), aztreonam (ATM), ertapenem (ETP), imipenem (IPM), TZP, MEM and tobramycin (TOB) (10). E. coli 25922 and PSA 27853 were utilized as quality control strains as recommended by CLSI. The inoculum of each organism was verified by colony counts. CLSI interpretative susceptibility criteria were utilized for each agent when available. In the absence of CLSI interpretative susceptibility criteria, the FDA breakpoints of 2 mg/L for Enterobacteriaceae and 4 mg/L for PSA were utilized for C/T. Susceptibility profiles were only defined for organisms with ≥30 isolates.
MDR PSA were classified as resistance to at least three classes represented by the following phenotypic resistance profiles: CIP (MIC ≥4 mg/L), IPM (MIC ≥8 mg/L), CAZ (MIC ≥32 mg/L), TZP (MIC ≥128 mg/L), and TOB (MIC ≥16 mg/L). ESBL phenotypic confirmation studies were undertaken for Enterobacteriaceae that had an initial positive screen test for ESBL production as defined by CLSI (5,10). Isolates testing non-susceptible (NS) to ETP (≥1 µg/mL), IPM (≥2 µg/mL) or MEM (≥2 µg/mL) underwent evaluation for carbapenemase production using the Carba NP test (11).
Results
Six US teaching hospitals provided 518 non-duplicate, consecutive isolates as follows: Enterobacteriaceae (n=275), PSA (n=144), Stenotrophomonas maltophilia (n=46), Serratia marcescens (n=32) and others as shown in Figure 1. Seventy-one percent of the organisms included in the current study were obtained from patients in the ICU.
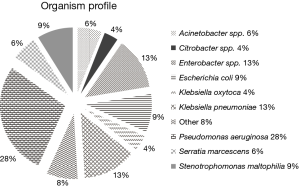
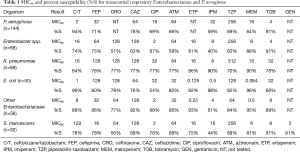
The concentration inhibiting 90% of the organisms (MIC90) and percent susceptibility (%S) for the Enterobacteriaceae and PSA are shown in Table 1. When considering the Enterobacteriaceae as a whole the carbapenems and C/T displayed the highest potency among the compounds tested. However, as noted the susceptibility profile of the individual compounds varied considerably among the subspecies of Enterobacteriaceae. While genotypic profiling was not undertaking for the organisms displaying MDR, phenotypic profiling did identify a total of 38 ESBL producing organisms as well as 33 Enterobacteriaceae defined as NS to the carbapenems, 9 of which were defined as having carbapenemases.
Full table
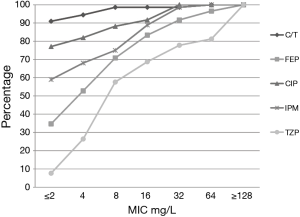
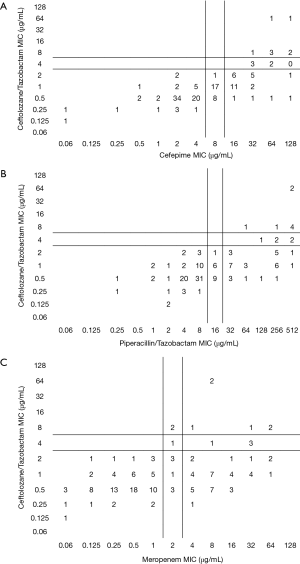
Among the PSA, C/T displayed the greatest degree of susceptibility at 94% (MIC90 =2 mg/L) followed by TOB 91% (4 mg/L) (Table 1). In comparison, the susceptibility profile for the remaining agents ranged from 59–78%, with their MIC90 values (mg/L) well in excess of the susceptibility breakpoint for all agents (MEM & CIP 16; FEP and IPM 32, ATM & CAZ 64 and TZP 256). As displayed in Figure 2, C/T demonstrated high potency as well as narrow MIC range against this population of nosocomial PSA. Moreover when the C/T MIC distribution of these PSA is compared to FEP, TZP or MEM using a scattergram assessment (Figure 3), the sustained susceptibility (i.e., MIC ≤4 mg/L) C/T is recognized despite increasing resistance to the other three β-lactams.
Fourteen percent of the PSA population was defined as MDR. In this subset of MDR isolates rank order %S (MIC90, mg/L) was as follows: C/T 75% [8], TOB 60% [64], ATM 15% [64], FEP 15% [64], CAZ 15% [128], CIP 5% [32], IPM 0% [32], MEM 0% [64], and TZP 0% [512]. The MIC90 was 8 for C/T, however, 45% of these organisms had MICs ≤1 mg/L, 15% =2 mg/L, 15% =4 mg/L, and 20% =8 mg/L.
When considering carbapenems, 41% and 36% of the PSA were NS defined as an MIC ≥4 mg/L to IPM and MEM, respectively. For this highly resistant population, C/T displayed the highest potency with 90% and 85% susceptibility for isolates NS to IPM and MEM. With the exception of TOB at 80% (MIC90, 64 mg/L), other agents demonstrated less than 54% susceptibility {CAZ 53% [128], FEP 49% [64], CIP 41% [32], TZP 41% [512], ATM 39% [64]} for this carbapenem NS population of PSA.
Within the PSA population 31% displayed a phenotypic profile of NS to TZP (MIC ≥32 mg/L). For these pseudomonal isolates, C/T demonstrated 82% susceptibility with 71% of the isolates having an MIC ≤2 mg/L. When considering those isolates defined as NS to FEP, C/T displayed 81% susceptibility (Figure 3).
Although less prevalent than the Enterobacteriaceae and PSA, S. maltophilia and Acinetobacter spp. are increasingly recognized pathogens in nosocomial pneumonia and thus MIC profiling was also undertaken for these organisms. While S. maltophilia and Acinetobacter spp. are not typically susceptible to the β-lactams utilized in the current study, we accessed these compounds inclusive of C/T in the context of empiric therapy (Table 2). While individual organisms displayed low and variable MICs profiles to the compounds investigated, the overall potency of the agents tested was poor with the exception of trimethoprim/sulfamethoxazole (TMP/SMX) for S. maltophilia.
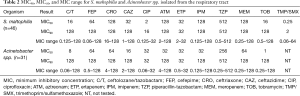
Full table
Since the initiation of treatment for nosocomial pneumonia is usually based on the development of signs and symptoms of infection (i.e., hypoxia, elevated white count, fever) and radiographic changes prior to the availability of culture data, the antibiotic(s) selected must provide potency against the pathogens of concern. In the setting of empiric therapy, Table 3 provides the probability of selecting a susceptible regimen consisting of either mono- or combination-therapy with TOB or CIP when considering the most prominent pathogens (i.e., Enterobacteriaceae and PSA) or the entire organism population (all bugs) as previously identified (n=518). When considering empiric monotherapeutic choices (i.e., β-lactams and CIP) for Enterobacteriaceae and PSA as well as all bugs, C/T was the most potent agent relative to other conventional therapeutic options. Although the additional of either CIP or TOB to all regimens improved the probability of having at least one appropriate antibiotic (i.e., susceptible) for these targeted respiratory pathogens the percent enhancement varied considerable based on the potency of β-lactams backbone selected.
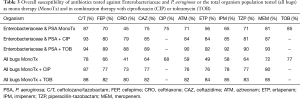
Full table
Discussion
Despite the implementation of preventive strategies to decrease the incidence of nosocomial infections including pneumonia, the underlying comorbid conditions of the host and the plethora of medical and surgical interventions in the ICU setting results in substantial infection risk for the patient. As a result of the increasing awareness of infection and its detrimental role in the care of the hospitalized patient, a corresponding increase in the utilization antimicrobials and resistance to conventional therapies has been noted at many institutions.
While good antimicrobial stewardship practices must be implemented in an effort to optimize the clinical utility of the current armamentarium, ongoing surveillance programs are necessary to identify the epidemiologic mix as well as the potency of the available antimicrobials in the institutional setting. In the current study we identified PSA as being the most prevalent organism in nosocomial-acquired respiratory tract cultures, an observation that is similar to previously conducted studies focusing on the epidemiology of hospitalized patients with pneumonia (12,13). Moreover, the beta-lactam phenotypic profile for this organism among these three studies conducted over the period of 2009–2014 reveals a longstanding concern regarding the potency of many parenteral agents as non-susceptibility rates range from 22% to 41%. Despite elevated levels of resistance to conventional parenteral beta-lactams and fluoroquinolones in our current study, C/T susceptibility was 94%, a value quite similar to the 93% previously reported by Farrell and colleagues from a 2012 collection of PSA (12).
While an assessment of in vitro potency is important once the organism(s) have been identified so that optimal directed therapy can be promptly administered, in the hospital setting the initiation of therapy is most often empirical and is based on the severity of illness as well as the local epidemiology and resistance profiles of the organism(s) of concern. Thus in consideration of empiric therapy for our currently derived nosocomial respiratory data set, the Enterobacteriaceae and PSA comprised 81% of all Gram-negative bacteria isolated. As a result it appeared reasonable to assess the susceptibility profile of this collective of organisms against potential therapeutic options. When cogitating monotherapy against the population of PSA and Enterobacteriaceae inclusive of those isolates producing ESBLs and carbapenemases, C/T displayed the greatest potency (87%) among the β-lactams. While the carbapenems are often considered to provide the most activity against Gram-negative pathogens, the increasing non-susceptibility PSA is resulting in an erosion of their overall potency. As might be expect when constructing the antibiogram inclusive of all the Gram-negatives respiratory isolates collected in our survey the susceptible of the agents tested dropped by 4–12% due predominately to the MDR of S. maltophilia and Acinetobacter.
While considerable debate has been levied regarding the clinical and microbiologic benefits of combination vs. monotherapy, one potential advantage is that combination therapy can increase the probability that at least one of the empiric agents will have activity against the target organism(s) (14,15). In our current study, when considering the Enterobacteriaceae and PSA population combination therapy enhanced the activity of antipseudomonal β-lactams by 6–27%. As a result of diminishing potency of the more frequently utilized β-lactams the largest increases in activity were seen with the addition of CIP or TOB to FEP, CAZ, ATM, TZP and IPM. Although enhancements in activity of up to 20% have been previously reported with combination therapy (16,17), it is important to recognized that TOB always produced greater activity than did CIP due to the increasing rate of intrinsic resistance to the fluoroquinolones. The current observation of the enhancement of susceptibility of the β-lactam-TOB combination is consistent with our longstanding appreciation of the synergistic effects of these two classes of antimicrobials (18). This advocacy for combination therapy in patients at risk for challenging pathogens such as PSA has been echoed in the recent Management of Adults with HAP/VAP Guidelines (19). While combination therapy might be utilized to enhance the microbiologic profile of the older β-lactams, institutional specific differences as noted in the current study between the use of CIP and TOB must also be noted when selecting combination therapy. While our data suggest that TOB provides a more robust enhancement of potency when used in combination, one must recognize that this is in the form of aminoglycoside monotherapy due to the non-susceptibility of the partner β-lactam. In addition, the potential risk associated with aminoglycosides therapy must be considered in any given patient. Conversely, our data suggest that if the goal of empiric therapy is to provide a high level of susceptibility for the target pathogens, newer agents such as C/T monotherapy will provide similar potency to either CIP or TOB combination therapy with the older β-lactams backbones.
It is widely recognized that appropriate antibiotic therapy, defined as the selection of an agent with in vitro potency against the target pathogen, is an important component to optimizing the clinical and microbiologic outcomes of patients with nosocomial infection (19,20). However, it is also well understood that the reduced antimicrobial potency of nosocomial respiratory pathogens as noted in this current study diminishes the clinical utility of many conventional agents because pharmacodynamic optimization may no longer be possible with standard dosing regimens (21). While dose escalation and/or alterations in the administration of β-lactams (i.e., prolonged or continuous infusions) are more frequently being utilized in an attempt to restore potency, the combination of host related pharmacokinetic factors (i.e., volume of distribution, enhanced drug clearance, poor penetration to the target site) and high-level resistance severely limits our ability to optimize drug exposures in an increasingly large population of critically ill patients. While these factors may reduce the clinical utility of older agents, the potency and pharmacokinetic profile of newer agents many provide innovative options for the management of these most difficult nosocomial respiratory tract infection clinical scenarios (8). As a result, it is of paramount importance to assess the potency of both the contemporary utilized agents as well as new therapies in an attempt to design antibiotic treatment strategies to optimize the probability of success.
Conclusions
The escalation of Enterobacteriaceae and PSA resistance among the most frequently used parenteral antibiotics has resulted in challenging clinical scenarios for the clinician and detrimental outcomes for patients. While combination therapy can be utilized to enhance the appropriateness of initial empire therapy, our data suggest that C/T monotherapy will provide a similar degree of microbiologic potency to combination regimens inclusive of the older less potent β-lactams. Awareness of one’s local resistance profiles, considerations of antibiotic pharmacodynamic optimization as well as the characteristics of newer therapies provides a sound foundation for the development of effective institutionally based stewardship programs.
Acknowledgements
The authors would like to thank Jennifer Hull, Lucinda Lamb, Sara Robinson, Elizabeth Cyr, and Debra Santini, for their collective efforts with MIC determination.
This study was supported by a grant from Cubist Pharmaceuticals, Lexington, MA. The sponsor of the study was not involved in the design, collection, analysis or interpretation of the data or in decision to submit the manuscript for publication.
Footnote
Conflicts of Interest: DP Nicolau is on the speaker bureau for Cubist and has received grants from Cubist. CA Sutherland has no conflicts of interest to declare.
References
- Dalhoff K, Ewig S, Gideline Development Group, et al. Adult patients with nosocomial pneumonia: epidemiology, diagnosis, and treatment. Dtsch Arztebl Int 2013;110:634-40.
- American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [Crossref]
- Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC Grand Rounds: Getting Smart About Antibiotics. MMWR Morb Mortal Wkly Rep 2015;64:871-3. [Crossref]
- ZERBAXA (ceftolozane/tazobactam) for Injection, for intravenous use. Initial U.S. Approval: 2014. Cubist Pharmaceuticals 2014. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206829lbl.pdf
- Sutherland CA, Crandon JL, Nicolau DP. Defining Extended Spectrum β-Lactamases: Implications of Minimum Inhibitory Concentration-Based Screening Versus Clavulanate Confirmation Testing. Infect Dis Ther 2015. [Epub ahead of print]. [Crossref]
- Bulik CC, Christensen H, Nicolau DP. In vitro potency of CXA-101, a novel cephalosporin, against Pseudomonas aeruginosa displaying various resistance phenotypes, including multidrug resistance. Antimicrob Agents Chemother 2010;54:557-9. [Crossref]
- Sutherland CA, Nicolau DP. Susceptibility Profile of Ceftolozane/Tazobactam and Other Parenteral Antimicrobials Against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa From US Hospitals. Clin Ther 2015;37:1564-71. [Crossref]
- Xiao AJ, Miller BW, Huntington JA, et al. Ceftolozane/tazobactam pharmacokinetic/pharmacodynamic-derived dose justification for phase 3 studies in patients with nosocomial pneumonia. J Clin Pharmacol 2016;56:56-66. [Crossref]
- ClinicalTrials.gov. Safety and Efficacy Study of Ceftolozane/Tazobactam to Treat Ventilated Nosocomial Pneumonia (MK-7625A-008) (ASPECT-NP). Available online: https://clinicaltrials.gov/ct2/show/NCT02070757
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. CLSI document M100-S24. Wayne, PA: Nat'l Comm Clinical Lab Standards, 2014.
- Dortet L, Poirel L, Nordmann P. Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. by using a biochemical test. Antimicrob Agents Chemother 2012;56:6437-40. [Crossref]
- Farrell DJ, Sader HS, Flamm RK, et al. Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012). Int J Antimicrob Agents 2014;43:533-9. [Crossref]
- Sader HS, Farrell DJ, Flamm RK, et al. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: results from the SENTRY Antimicrobial Surveillance Program, 2009-2012. Int J Antimicrob Agents 2014;43:328-34. [Crossref]
- Durante-Mangoni E, Grammatikos A, Utili R, et al. Do we still need the aminoglycosides? Int J Antimicrob Agents 2009;33:201-5. [Crossref]
- Leibovici L, Vidal L, Paul M. Aminoglycoside drugs in clinical practice: an evidence-based approach. J Antimicrob Chemother 2009;63:246-51. [Crossref]
- Bhat S, Fujitani S, Potoski BA, et al. Pseudomonas aeruginosa infections in the Intensive Care Unit: can the adequacy of empirical beta-lactam antibiotic therapy be improved? Int J Antimicrob Agents 2007;30:458-62. [Crossref]
- Beardsley JR, Williamson JC, Johnson JW, et al. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest 2006;130:787-93. [Crossref]
- Owens RC Jr, Banevicius MA, Nicolau DP, et al. In vitro synergistic activities of tobramycin and selected beta-lactams against 75 gram-negative clinical isolates. Antimicrob Agents Chemother 1997;41:2586-8.
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111. [Crossref]
- Bhalodi AA, Nicolau DP. Principles of Antimicrobial Therapy and the Clinical Pharmacology of Antimicrobial Drugs. In: Hall JB, Schmidt GA, Kress JP. editors. Principles of Critical Care. 4th edition. New York: McGraw-Hill Education, 2015;544-51.
- Monogue ML, Kuti JL, Nicolau DP. Optimizing Antibiotic Dosing Strategies for the Treatment of Gram-negative Infections in the Era of Resistance. Expert Rev Clin Pharmacol 2016;9:459-76. [Crossref]


