Tracheobronchial tuberculosis: a clinical review
Introduction
Tuberculosis (TB) remains a heavy global health burden. The 2015 Global WHO TB report mentions an estimated 9.6 million incident TB cases, which was a considerable increase compared to estimates from 2014 (1). Of TB cases, patients with endobronchial tuberculosis (EBTB) often face a delayed diagnosis. EBTB is defined as a tuberculous infection involving the tracheobronchial tree. Its exact pathogenesis is unclear. However, postulations include: (I) direct infiltration of disease from the lungs; (II) infected sputum/secretions causing direct implantation of organisms; (III) haematogenous spread; (IV) lymphatic dissemination; and (V) erosion of lymph nodes into the trachea or bronchus (2). EBTB has complex and varying clinical courses and continues to bear a heavy health burden due to the severe sequelae of bronchostenosis.
Clinical and radiological features
EBTB appears to have a preponderance in females in their second and third decades of life (3,4). Van de Brande et al. have described EBTB in an elderly population as well with a mean age of 70 years (5). Symptoms include a productive cough, chest pain, haemoptysis, lethargy, fever and dyspnea (3). Clinical findings are heterogeneous, and can include a focal wheeze and decreased air entry on auscultation (3). Because the signs and symptoms are non-specific, the diagnosis of EBTB should be made with a combination of clinical suspicion, clinical findings, and radiology and sputum/tissue analyses.
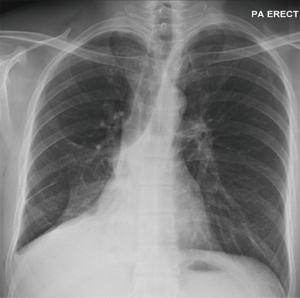
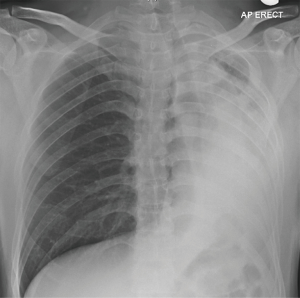
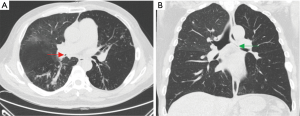
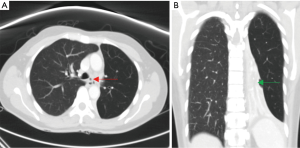
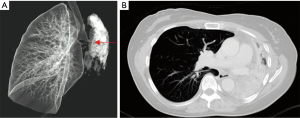
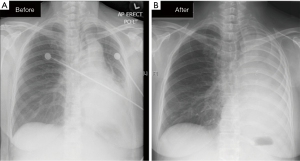
Chest roentgenograms (CXR) may appear normal unless there is significant airway obstruction leading to atelectasis of the distal pulmonary segment, or concurrent parenchymal or pleural disease (Figures 1,2). Computer tomography (CT) of the thorax would yield more detail such as irregularities/stenosis of the airways, as well as other features of TB in the thorax such as mediastinal lymphadenopathy, nodules, cavities and pleural effusions (Figures 3,4). Furthermore, a 3-dimensional (3D) reconstruction of the airways (Figure 5) allow for estimating the extent of airway narrowing, and help plan for further intervention such as bronchoscopy as well as surgery (6).
Contrary to clinical expectations, sputum analysis in EBTB has variable diagnostic yield, with reports from 17% to 79% if combined with specimens obtained via bronchoscopy (7,8). Postulations to this include the lack of ulceration in the mucosal wall of the bronchus, or difficulties in expectoration (9). As such, adjunct methods such as nucleic amplification tests are now increasingly used to help detect TB and aid in more rapid diagnosis (10).
Endoscopic and histopathologic findings
EBTB tends to affect the right upper and right main bronchi (7), though any part of the tracheobronchial tree can be afflicted. It is well-described to take on different endoscopic appearances. A comprehensive study done by Chung et al. has described the following subtypes: (I) actively caseating (Figure 6A); (II) oedematous-hyperaemic (Figure 6B); (III) fibrostenotic (Figure 6C); (IV) tumorous (Figure 6D); (V) granular (Figure 6E); (VI) ulcerative (Figure 6F); and (VII) nonspecific bronchitis (Figure 6G) (10). Interestingly, the tendency to develop bronchial stenosis could be predicted from bronchoscopic appearances of the mucosa. Oedematous-hyperaemic, fibrostenotic, and tumorous subtypes tended to progress to eventual bronchial stenosis/obstruction within 3 months despite appropriate treatment (10). On analyzing bronchial lavage fluid samples, the granular subtype seemed to yield highest smear and culture positivity for Mycobacterium tuberculosis, whereas both tests were negative for the fibrostenotic and nonspecific subtypes (11). The presence of caseating granulomas or acid fast bacilli (AFB) would cement the diagnosis of EBTB.
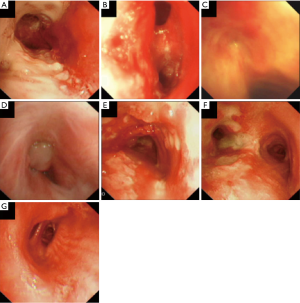
The above classification of EBTB can be explained by evolution of the disease. The initial lesion, with lymphocytic infiltration of the submucosa, is characterized by simple erythema and oedema of the bronchial mucosa. This would correspond to the nonspecific bronchitis subtype. With the formation of submucosal tubercles, a more granular appearance develops (the granular subtype). Subsequent oedema and erythema gives rise to the oedematous-hyperaemic subtype. At this stage, the oedema can result in bronchial stenosis. From here, the lesion can undergo caseous necrosis with tuberculous granuloma formation on the mucosa (leading to the actively caseating appearance). If the inflammation persists and breaches the mucosa, an ulcer is formed, giving rise to the ulcerative subtype. Rikimaru et al. further described three stages of bronchial ulcers (12): (I) active stage or Stage A which were only observed before TB treatment commenced; (II) healing stage (Stage H) and scarring (Stage S). The mucosal ulcer can further evolve to form hyperplastic, inflammatory polyps, forming the tumorous subtype. Ultimately, the endobronchial lesions would heal via fibrostenosis (6,11).
Management
Interactions of EBTB with host immunity, antituberculous treatment and the effects of mycobacterial infection lead to a heterogenous clinical course.
For active disease, the two goals of treatment are eradication of the tuberculous bacilli, as well as mitigating development of tracheobronchial stenosis, although stenosis can occur despite timely treatment with antituberculous drugs (7). A systematic review and meta-analysis by Critchley et al. showed that steroids could be effective in mortality reduction in all forms of TB (13). Nemir et al. (14) found prednisone as an advantageous addition to lymph node TB therapy if given within four months of the course of illness. However, specific to EBTB, the routine use of corticosteroids remains controversial. Shim (15) proposed steroid therapy for the oedematous-hyperaemic, caseating and tumorous subtypes. The suggested dose was prednisolone 1 mg/kg for 4–6 weeks followed by slow taper over a further 4–6 weeks. Conversely, a prospective study of 34 adult patients using corticosteroids in the treatment of EBTB showed no significant difference in healing rates that were observed bronchoscopically, and that treatment did not affect pre-existing fibrotic lesions (16). Similarly, a retrospective study by Um et al. (17) did not find additional benefit in the addition of prednisolone to prevent airway stenosis for patients with proven EBTB.
Patients who suffer from bronchial stenosis leading to distal obstruction and atelectasis often present with wheezing (which can be localised on auscultation) and dyspnoea. Treatment modalities to restore patency of the airways include bronchoplasty and surgery.
Bronchoplasty via interventional bronchoscopy allows for the application of various techniques such as balloon dilatation (Figures 7A,B), argon plasma coagulation, laser, electrosurgery and cryotherapy; these methods can be applied either singly or in combination to achieve the desired results (8,18-20). Cho et al. (19) performed balloon dilatations on 113 EBTB patients with tracheobronchial strictures and reported a 73% success rate, after single or multiple dilatations.
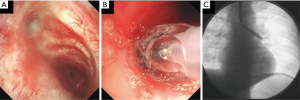
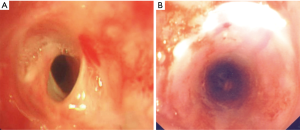
Various factors can influence the success of balloon dilatation. Cho et al. (19) encountered treatment failure in 27% (n=31) of patients who had undergone balloon dilatation, with recurrence of symptoms between 1 day to 113 months with a mean of 13 months. All patients had been receiving antituberculous therapy for a minimum of 5 months. For the patients who failed to respond to balloon dilatation, alternate measures such as a temporary stent, cutting balloon dilatation, radiation-eluting balloon dilatation or surgical measures were explored. He found a longer stricture length tended towards a shorter patency period post balloon dilatation, and suggested using a larger balloon diameter (up to 12 mm for bronchial strictures and 20 mm for tracheal strictures). Silicon stents (Figure 8) can be inserted into the airway once recanalised to maintain patency, whereas metallic stents should be used very cautiously due to overgrowth of granulation tissue as well as mechanical complications such as stent fracture (19-22). Other complications related to balloon dilatation as well as stenting include airway wall perforation, stent migration leading to obstruction, and haemoptysis (21).
A retrospective study looking at CT features as predictors of outcome after endobronchial interventions (including balloon dilatation, stenting and surgical bronchoplasty) in patients with EBTB found the presence of parenchymal calcification, and bronchiectasis within atelectasis had a higher tendency towards failure. Interestingly, mucus plugging, extent of airway narrowing and volume loss on CT images did not affect patient outcomes after expansion procedures (23).
Surgery is considered when interventional bronchoscopic measures fail. Lobectomy or pneumonectomy has been well-established (Figure 9). Other methods such as sleeve resection, carinal resection, reconstruction of the bronchus or trachea and end-to-end anastomosis have been used to treat post-tuberculous airway stenosis (24-26).
Conclusions
EBTB presents with non-specific, insidious symptoms. There must be a high index of clinical suspicion, and its diagnosis should be supported by radiological and bronchoscopic appearances as well as microbiological/histopathological evidence. The mainstay of treatment remains to be antituberculous chemotherapy. The role of corticosteroids is controversial, and even with timely treatment, some patients eventually progress to tracheobronchial stenosis. CT with 3D reconstruction of the airways allow for better planning of bronchoscopic or surgical procedures. Bronchoscopic-guided balloon dilatation can be done as a single procedure, or may require repeated staged events, and might be used in combination with other tools such as electrocautery, argon plasma coagulation, laer, or cryotherapy. Stents, preferably silicone stents, may be inserted to maintain airway patency. Should these measures fail, surgical options can be explored to help patients with debilitating stenosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organisation. Global tuberculosis report 2015. Available online: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf
- Smart J. Endo-bronchial tuberculosis. Br J Tuberc Dis Chest 1951;45:61-8. [Crossref]
- Lee JH, Park SS, Lee DH, et al. Endobronchial Tuberculosis: Clinical and Bronchoscopic Features in 121 Cases. Chest 1992;102:990-4. [Crossref]
- Jung SS, Park HS, Kim JO, et al. Incidence and clinical predictors of endobronchial tuberculosis in patients with pulmonary tuberculosis. Respirology 2015;20:488-95. [Crossref]
- Van den Brande PM, Van de Mierop F, Verbeken EK, et al. Clinical Spectrum of Endobronchial Tuberculosis in Elderly Patients. Arch Intern Med 1990;150:2105-8. [Crossref]
- Lee P. Endobronchial tuberculosis. Indian J Tuberc 2015;62:7-12. [Crossref]
- Ip MS, So SY, Lam WK, et al. Endobronchial tuberculosis revisited. Chest 1986;89:727-30. [Crossref]
- Kashyap S, Solanki A. Challenges in Endobronchial Tuberculosis: From Diagnosis to Management. Pulm Med 2014;2014:594806.
- Kashyap S, Mohapatra PR, Saini V. Endobronchial Tuberculosis. Indian J Chest Dis Allied Sci 2003;45:247-56.
- Chung HS, Lee JH. Bronchoscopic Assessment of the Evolution of Endobronchial Tuberculosis. Chest 2000;117:385-92. [Crossref]
- Ozkaya S, Bilgin S, Findik S, et al. Endobronchial tuberculosis: histopathological subsets and microbiological results. Multidiscip Respir Med 2012;7:34. [Crossref]
- Rikimaru T, Tanaka Y, Ichikawa Y, et al. Endoscopic classification of tracheobronchial tuberculosis with healing processes. Chest 1994;105:318-9. [Crossref]
- Critchley JA, Young F, Orton L, et al. Corticosteroids for prevention of mortality in people with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:223-37. [Crossref]
- Nemir RL, Cardona J, Vaziri F, et al. Prednisone as an adjunct in chemotherapy of lymph node-bronchial tuberculosis in childhood: a double-blind study. Am Rev Respir Dis 1967;95:402-10.
- Shim YS. Endobronchial tuberculosis. Respirology 1996;1:95-106. [Crossref]
- Park IW, Choi BW, Hue SH. Prospective study of corticosteroid as an adjunct in the treatment of endobronchial tuberculosis in adults. Respirology 1997;2:275-81. [Crossref]
- Um SW, Yoon YS, Lee SM, et al. Predictors of persistent airway stenosis in patients with endobronchial tuberculosis. Int J Tuberc Lung Dis 2008;12:57-62.
- Low SY, Hsu A, Eng P. Interventional bronchoscopy for tuberculous tracheobronchial stenosis. Eur Respir J 2004;24:345-7. [Crossref]
- Cho YC, Kim JH, Park JH, et al. Tuberculous Tracheobronchial strictures Treated with Balloon Dilation: A Single-Center Experience in 113 Patients during a 17-year Period. Radiology 2015;277:286-93. [Crossref]
- Lee KW, Im JG, Han JK, et al. Tuberculous stenosis of the left main bronchus: results of treatment with balloons and metallic stents. J Vasc Interv Radiol 1999;10:352-8. [Crossref]
- Lee P, Kupeli E, Mehta AC. Airway stents. Clin Chest Med 2010;31:141-50. Table of Contents. [Crossref]
- Iwamoto Y, Miyazawa T, Kurimoto N, et al. Interventional bronchoscopy in the management of airway stenosis due to tracheobronchial tuberculosis. Chest 2004;126:1344-52. [Crossref]
- Lee JY, Yi CA, Kim TS, et al. CT scan features as predictors of patient outcome after bronchial intervention in endobronchial TB. Chest 2010;138:380-5. [Crossref]
- Lei Y, Tian-Hui Z, Ming H, et al. Analysis of the surgical treatment of endobronchial tuberculosis (EBTB). Surg Today 2014;44:1434-7. [Crossref]
- Natkunam R, Tse CY, Ong BH, et al. Carinal resection for stenotic tuberculosis tracheitis. Thorax 1988;43:492-3. [Crossref]
- Nakamoto K, Maeda M. Tracheobronchoplasty for endobronchial tuberculosis. Kekkaku 1991;66:789-92.