In vitro activation of hTERT-specific T cell responses in lung cancer patients following chemotherapy
Introduction
Patients with advanced non-small cell lung cancer (NSCLC) have an unfavourable prognosis. Although recent therapeutic advances using tyrosine kinase inhibitors enabled improved prognosis—particularly for patients with epidermal growth factor receptor (EGFR) mutations—most patients still show an unsatisfactory median survival of only 8 months (1).
In addition to current radio- and chemotherapy protocols, tumor antigen-specific T cell mediated immunotherapy is widely accepted as an attractive therapeutic strategy. In particular, CD8+ cytotoxic T lymphocytes (CTL) are considered to be major mediators of tumor immuno-surveillance (2,3) recognising tumor-associated antigens (TAA) as cognate peptides bound to major histocompatibility molecules expressed on the surface of tumor cells. A specific T cell receptor (TCR) binds to these peptide-MHC (pMHC) complexes causing CTL to proliferate, produce cytokines, detect and mark target cells presenting the same antigen for lysis. Both active and passive immunotherapies using TAA-specific T cells have already demonstrated their efficiency in a number of clinical studies (4-6).
Human telomerase reverse transcriptase, hTERT, has been identified as the catalytic enzyme required for telomere elongation (7) and is expressed in 93-100% of NSCLC (8,9). Former studies have already demonstrated that peptides derived from hTERT are (I) naturally processed by tumors; (II) are presented on MHC molecules and (III) trigger effector functions of specific CTL (10-12).
Clinical trials of multiple vaccine formulations have illustrated that hTERT-specific immune responses can be safely induced in cancer patients and have a noticeable impact on clinical results. In HLA-A2+ women with metastatic breast cancer who were subcutaneously vaccinated with the hTERT I540 peptide, an exploratory landmark analysis revealed an association between a hTERT-specific CD8+ T cell immune response and overall survival (13). In another study, patients with non-resectable pancreatic cancer were immunized with the E611 peptide (14). The authors of this study were able to demonstrate that median survival of patients exhibiting an immune response (termed “responders”) was significantly higher than that of those who did not respond to the immunization (termed “non-responders”). To test the immunogenicity of low-affinity hTERT peptides, subcutaneous injections of 572Y modified peptide were applied to HLA-A2+ patients with advanced NSCLC. The estimated overall survival was 30 months for immunological responders vs. 4 months for non-responders (15) demonstrating lengthier survival in patients with a hTERT-specific immune response.
Despite these recent technological advances in vaccination, the number of patients showing an immune response to the hTERT-immunization is still limited. Currently, second-generation vaccines are addressing strategies to enhance cellular immunity against hTERT without toxicity. In vitro the stimulatory effects of DC can be initiated either by the well-established cytokine cocktail or by Toll like receptors (TLR), which have an essential function in recognition of microbial and viral infections (16). An additional improvement of TAA-specific T-cell responses can be achieved by triggering multiple immunological pathways. Using the TLR7/8-agonist R848 as a priming signal in combination with a soluble (s) CD40-ligand (L) as a maturation signal resulted in the induction of an intensive expression of IL-12p35 and p40 in myeloid DC. IL-12 polarized CD4+ T cells to Th1 cytokine production and induced CD8+ T cells with high functional avidity and tumor cell recognition (17).
In murine models as well as in clinical studies, prior host immunosuppression can dramatically improve the anti-tumor effect of both, adoptive T cell transfer (18) and vaccination (19) protocols. Lymphodepleting but non-myeloablative chemotherapy prior to adoptive T cell transfer provides space for transferred lymphocytes and allows their clonal host re-establishment. Lymphodepletion may also decrease the number of regulatory T cells (Treg), which are strongly suspected to interfere with the activation of TAA-specific immune cells. Regulatory T cells prevent the expansion of tumor-reactive T cells in vitro. In patients with hematologic malignancies the depletion of Treg from PBMCs enabled the rapid expansion of WT1-specific CTL in vitro, whereas no CTL could be generated from unfractionated PBMC (20). In seronegative cancer patients in vitro induction of NY-ESO-1 specific Th1 cells required depletion of CD25+ T-cells (21). The analysis of four clinical trials employing non-myeloablative chemotherapy—with or without total body irradiation (TBI)—prior to adoptive T cell transfer revealed that the percentage and number of reconstituting CD4+FoxP3+ Tregs observed in the peripheral blood was higher in non-responders than responders. These observations provide strong evidence that endogenous CD4+ Tregs have a negative impact on cancer immunotherapy (18). Detailed studies of CD4+CD25+Foxp3+ Tregs cells in 104 individuals affected with ovarian carcinoma have shown that human tumor Tregs suppress tumor-specific T cell immunity and contribute to growth of human tumors in vivo. Tumor Tregs are also associated with a high death hazard and reduced survival (19).
In this study hTERT-specific T cell responses were activated in vitro in PBMC of patients with advanced NSCLC. Soluble CD40L in combination with the TLR7/8-agonist CL097, a highly water-soluble derivative of the imidazoquinoline compound R848, was used to improve activation of hTERT-specific T-cell responses following depletion of CD4+CD25+ regulatory T cells. This approach was tested in patients with advanced NSCLC exhibiting different HLA types who simultaneously received platinum based standard 1st line chemotherapy.
Materials and methods
Patients
All patients were treated with standard 1st line platinum containing chemotherapy regimes at the University Medical Center Schleswig-Holstein. After informed consent, peripheral blood samples from 7 patients (A-G) with advanced NSCLC (Table 1) were obtained between chemotherapy cycles. Corresponding HLA-typing of the peripheral blood mononuclear cells (PBMC) was performed by PCR.
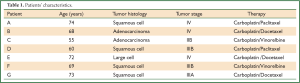
Full Table
Isolation of PBMC and generation of DC
PBMCs were isolated by centrifugation on a Ficoll-Paque plus (GE Healthcare Bio-Sciences AB, Munich, Germany) density gradient from peripheral blood samples. DCs were obtained as previously described (22) with minor modifications. Briefly, PBMCs were resuspended at 2×106-2×107 cells/mL in 10 mL CellGroDC (CellGenix, Freiburg, Germany) and incubated for 2 h in a 250 mL plastic flask. The non-adherent lymphocytes (PBL) were removed and frozen in 50% RPMI 1640 (Biochrom, Berlin, Germany) supplemented with 40% heat-inactivated pooled human AB serum (Baxter, Unterschleissheim, Germany) and 10% DMSO according to standard protocols. The adherent fraction was cultivated in presence of 800 U/mL GM-CSF and 500 U/mL IL-4 (R&D, Wiesbaden, Germany). Fresh medium supplemented with cytokines was added on days 3 and 5. On day 6, floating cells were harvested and used for antigen loading.
DC pulsing with hTERT peptides and maturation protocols
For activation of HLA-A and HLA-DR-restricted T cell responses hTERT peptides (Table 2) were used to load stimulator cells. 30 μg of each hTERT peptide were added to the DCs (3×106) in 1mL CellGro supplemented with 500 U/mL IL-4 and 800 U/mL GM-CSF. DCs were incubated at 37 °C for 2 h. For DC maturation a terminal differentiation-inducing standard cytokine cocktail consisting of 2.000 IE/mL IL-1β, 1,000 IE/mL IL-6, 1,000 IE/mL TNF-α (all: R&D Systems, Wiesbaden, Germany) and 1 μg/mL prostaglandin E2 (Sigma Aldrich, St. Louis, USA) was added and the culture was incubated overnight (26). For comparison, T cells were treated with 1 μg/mL CL097 (InvivoGen, San Diego, USA) for 2 h and matured with 1 μg/mL sCD40L (Miltenyi, Bergisch-Gladbach, Germany) for an additional 20 h. Subsequently, DCs were washed and used for stimulation of autologous T cells.

Full Table
Activation of hTERT-specific T cell responses
Prior to T cell culture non-adherent PBMCs from 7 patients (A-G, Table 1) were depleted of CD4+CD25+ Tregs using the CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec, Bergisch-Gladbach, Germany) according to the manufacturer’s instructions. CD4+ cells were separated with a biotinylated CD4 cell antibody cocktail. Following positive selection of CD4+CD25+ cells with CD25 MicroBeads, the CD4- fraction and the CD4+CD25- population were co-cultured in vitro with hTERT peptide-pulsed DCs using a protocol adapted from a previous study (22). Briefly, 1×106 non adherent PBMC depleted of T regs were cultured in RPMI 1640 and 2 mM L-Glutamine (both Biochrom, Berlin, Germany), 5% AB-Serum (Baxter, Unterschleissheim, Germany), 50 μg/mL Gentamicin (GIBCO, Invitrogen, Darmstadt, Germany) with hTERT-pulsed DC (1×105). After 7 days in culture, a second stimulation was performed followed by addition of 20 IU/mL IL-2 (R&D Systems, Wiesbaden, Germany) on day 10. After 14 days in culture, a third stimulation was performed. After a total of 15 days in culture, the peptide-stimulated T cells were tested for specificity by intracellular IFN-γ FACS.
Intracellular cytokine staining
To assess the capacity of T cells to produce IFN-γ upon recognition of a specific target, intracellular IFN-γ staining was performed. Target cells used in both the experimental and the standard groups included hTERT-peptide-loaded DCs. 2 h after start of stimulation 1 µL/mL Brefeldin A (Sigma-Aldrich, St. Louis, USA) was added to the cultures. After 10 h cells were fixed and permeabilized with Inside Fix and Inside Perm (BD Biosciences, San Jose, USA) and stained with the following intracellular and cell surface markers: PE-Cy5-CD3, PE-CD8, PerCP-Cy5, 5-CD4 and ECD-CD45 (Beckman Coulter, Krefeld, Germany) and IFN-γ-FITC (Miltenyi Biotec, Bergisch-Gladbach, Germany). Samples were analyzed on a FACS-Calibur flow cytometer. Gating for cytokine was based on a positive T cell control sample which had been stimulated with staphylococcal enterotoxin B (SEB) or staphylococcal enterotoxin A (SEA) (Sigma-Aldrich, St. Louis, USA). T cells incubated with non-peptide loaded DC were used as negative controls. CELLQuest software (Becton Dickinson, San Jose, USA) was used for analysis of T cell responses detected by multi-color flow cytometry and generation of graphical representations. Background subtraction was performed for all samples used for analyzing proportionate representation of responses.
Cytotoxicity assay
T cells were tested for specific cytotoxicity against T2 cells loaded with hTERT peptides. Target cells (1×106) were labeled with 0.1 mCi 51Cr and mixed with various numbers of effector cells to give E:T ratios of 40:1, 20:1, 10:1 and 5:1. Target cells incubated in complete medium or 5% Triton X-100 (Sigma) were used to determine spontaneous and maximal 51Cr release, respectively. After 4 h, supernatants were collected and radioactivity was measured on a gamma counter. The mean percentage of specific lysis of triplicate wells was calculated as 100× (experimental release-spontaneous release)/(maximal release-spontaneous release).
Results
Expression of surface maturation markers on dendritic cells
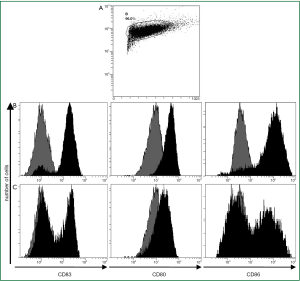
Maturation and costimulatory molecules like CD80, CD83 and CD86 are essential for DCs to induce an effective cytotoxic T-lymphocyte (CTL) response. Because alteration in surface protein expression is an important step in the maturation of DCs, we investigated whether the TLR7/8-agonist in combination with sCD40L had an additive effect on the expression of DC costimulatory molecules.
After culture in GM-CSF and IL-4 adherent PBMCs showed typical DC morphology including dendrites extending in several directions. Based on forward-side-scatter dot plots these large granular cells were gated as DC (Figure 1A). The immature DCs were either activated by the standard cytokine cocktail (cDC) or first primed for 2 h with the CD7/8-agonist and then matured for an additional 20 h with the sCD40L (ligDC). The PBMC-derived large granular cells showed upregulation of CD80, CD86 and CD83 indicating maturation of DCs. However DCs activated by the TLR7/8-agonist and sCD40L displayed lower levels of costimulatory molecules than DCs matured by the standard cytokine cocktail. Almost all DCs matured by the cytokine cocktail upregulated CD80, CD83 and CD86 (Figure 1B), but only 50% of DCs that had been matured by the TLR7/8-agonist and sCD40L upregulated CD83 and CD86 (Figure 1C). Though CD80 was upregulated in all DCs, this surface marker was less intensely expressed in the DCs matured by the TLR7/8-agonist and sCD40L. These results are representative for 5 experiments performed in patients A, B, C, D and G. The findings show that the expression of surface markers analyzed in this study is more efficiently induced by the cytokine cocktail than by TLR7/8-agonists combined with sCD40L.
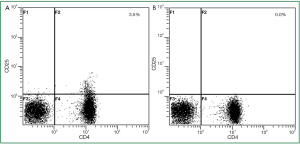
Depletion of regulatory T cells
As regulatory T cells prevent in vitro expansion of tumor-reactive T cells, we first depleted CD4+CD25+ regulatory T cells in non-adherent PBMC. As examplified in HLA-A*02 patient G, depletion of regulatory T cells resulted in 0% CD4+CD25+ regulatory T cells (Figure 2). In 3 analyzed patients this appoach resulted in a mean of 0.1% regulatory T cells in the non-adherent PBMC being subsequently stimulated with hTERT-peptide loaded DC.
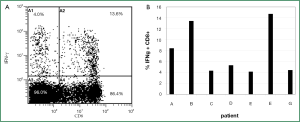
Activation of hTERT-specific T cell responses
Before analysis of hTERT-specific T cell reactivity a positive control for the T cells’ ability to produce cytokines was performed. The T cell responses to the strong immunogenic stimuli SEA and SEB, respectively, were analyzed on non-adherent, Treg depleted PBMC of all NSCLC patients (Figure 3A). The specific IFN-γ production of CD8+ T cells was detected within a range of 4.1% to 14.7% and a median of 7.8% (Figure 3B).
Detection of hTERT-specific cellular immune responses was performed on non-adherent, Treg depleted PBMC of NSCLC patients after in vitro stimulation with autologous hTERT-peptide loaded DC. The peptides used for DC pulsation were selected following published data after MHC class I and II complex analysis (Table 3). Recognition of the HLA-matched hTERT peptides by non-adherent, Treg depleted PBMC was analyzed using functional flow cytometry of the peptide-induced intracellular IFN-γ production. Target cells used in both the experimental ligDC and the standard cDC groups included hTERT-peptide-loaded DCs. Non-peptide-loaded DCs were used as negative controls. The percentage of hTERT-specific T cells was calculated by substraction of IFN-γ+ T cells in absence of antigen (negative control) from the number of IFN-γ+ T cells in presence of hTERT-peptide loaded DC.

Full Table
With DC that had been matured with the standard cytokine cocktail hTERT-specifc IFN-γ production of CD8+ T cells could be induced in PBMC samples of 4 (A-D) out of 7 patients. Using this standard approach, 0.5-1.5% IFN-γ+ CD8+ T cells could be activated (Figure 4).
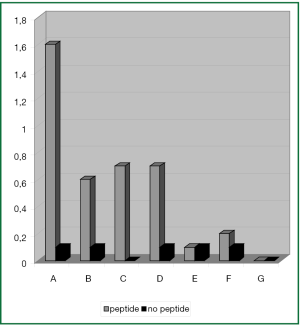
Interestingly, the most intense T cell response was observed in PBMC samples of patient A. Due to the patient’s HLA-type the DCs were loaded with both the HLA-A2 restricted I540 and the HLA-A24 restricted V461 hTERT-peptide.
In 1 additional patient (F) only a very small population of T cells could be activated comprising of 0.1% T cells with hTERT-reactivity.
Since there is strong correlation between the intracellular IFN-γ cytokine-staining assay and the 51Cr-release assay we used the intracellular cytokine staining in the present study as a surrogate marker for cytolytic activity (23). To confirm the function of the activated IFN-γ+ hTERT-specific T cells a cytotoxicity assay was done in patient F. However, in this patient T lymphocytes that had been stimulated with hTERT-peptide pulsed cDC did not lyse hTERT-peptide loaded T2 target cells (data not shown).
In the remaining 2 patients (E, G) immunophenotypic analysis of PBMC samples could not reveal any activation of hTERT-specific IFN-γ+ T cell responses after stimulation with cytokine matured DC.
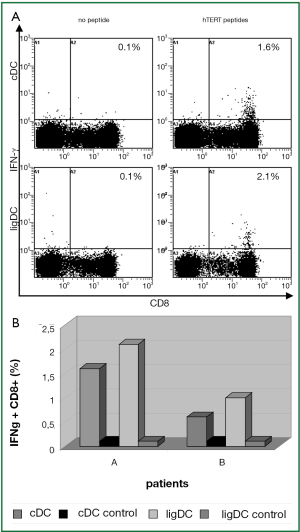
In 2 patients (A, B) in whom the hTERT-specific T cell response had already been activated by the standard cytokine approach, the reaction to hTERT could be further intensified by TLR7/8-agonist and sD40L matured DCs resulting in 0.9% and 2.0% IFN-γ+CD8+ T cells, respectively (Figure 5A,B).
In PBMC samples of patient C and patient E a small population of 0.1% IFN-γ+ CD8+ T cells could be activated by hTERT-peptide loaded ligDC (Figure 6). This population could not be expanded and functionally tested. Lacking functional data we cannot say if this CD8+ T cell population is recognizing hTERT expressing target cells.
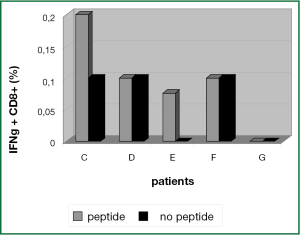
In PBMCs of patients D, F and G there was no hTERT-specific activation signal after stimulation of T cells with hTERT peptide loaded ligDC (Figure 6).
Taken together, hTERT-specific T cells could be activated in PBMC samples of 4 patients by usage of the standard cytokine cocktail. In 2 of these patients the percentage of hTERT-specific T cells could be further increased by DC that had been matured with TLR7/8-agonist and sCD40L.
Additional functional assays were prevented by the insufficient T cell numbers available. Although in the present study hTERT-specific T cells were activated, the total number of T cells decreased. Starting with a median of 6×106 T cells, there was a loss of CD8+ cells resulting in a median of 1.3×106 T cells after 15 days of culture.
Discussion
In present vaccination and adoptive T cell transfer studies activation of cancer-specific T cell responses is associated with a favourable prognosis. In HLA-A2+ patients with advanced NSCLC the estimated overall survival after vaccination with hTERT peptides was 30 months for immunological responders vs. 4 months for non-responders (15) demonstrating a survival advantage in patients with a hTERT-specific immune response. Since the induction of tumor-specific CD8+ T cell response correlates with anti-tumor efficiency, improved protocols for the activation of tumor-specific CD8+ T cell responses are urgently needed.
Regulatory T cells suppress tumor-specific T cell responses and thereby play a key role in cancer immune escape. In patients with metastatic urothelial carcinoma the NYESO-1 directed T cell responses were augmented by Treg depletion (27). Also in cancer patients WT1-specific CD8+ T cell responses could be activated in vitro only after depletion of Tregs (20). In accordance with these data in the present study we have stimulated PBMC with peptide loaded DC after depletion of Treg. Following the depletion of regulatory T cells hTERT-specific T cell reactivity could be generated. However, CD25 is also expressed on activated CD4+ T cell subsets. Because of that the depletion of CD4+CD25+ T cells potentially impairs the CD4+ helper T cell response leading to a decreased activation of the hTERT-specific CD8+ T cells. This might especially be important in those patients in whom the non-adherent PBMC were stimulated with MHC class II restricted peptides.
Boosting pre-existing, naturally occurring CD8+ T cell responses directed against class-I MHC-restricted peptides of tumor antigens, represents a primary goal of both active and passive immunotherapy. In a previous study the frequency of circulating hTERT-specific T cells detected in lung cancer patient upon diagnosis was 1×10-7-42×10-7 peripheral blood CD8+ cells (28). In the present study, hTERT-specifc IFN-γ production of CD8+ T cells could be induced in vitro by different DC maturation cocktails in PBMC samples of 4 out of 7 patients with advanced NSCLC. In these patients, hTERT-specific T cell responses between 0.5% and 2.1% could be activated potentially indicating expansion of base line hTERT-specific T cells. This expansion process could be monitored by hTERT-tetramers.
These data are in accordance with other groups aiming at the activation of hTERT-specific T cells. Filaci et al. (24) demonstrated I540-specific activation of CD8+ T cell responses in patients with different types of cancer after in vitro stimulation with peptide-loaded antigen presenting cells (APC). Generation of p540 peptide-specific CTL lines was tested in 16 cancer patients, but CTL lines could be generated in 11 patients (68%) only, in particular from 8 of 12 prostate cancer patients, 1 of 2 lung cancer patients and 2 patients affected with gastric and liver cancer, respectively (24).
In patients with multiple myeloma a single ex vivo peptide stimulation led to activation of 0.3-0.5% of hTERT-specific cells, but this effect occurred to be limited to only 2 of 27 patients (25).
In 6 out of 8 lymphoma and solid tumor patients 0.5% I540-specific CD8+ cells were identified after three in vitro stimulations of PBMC with peptide loaded APC. Four ex vivo stimulations were necessary to identify 1-3% I540-specific CD8+ T cells (29).
Though we and others have reproducibly activated TAA-specific T cell using dendritic cells as APCs (22) in all other studies mentioned above different types of peptide loaded APC were used. All approaches were finally activating similar quantities of hTERT-specific T cells. Since the generation of DC is both time consuming and laborious the use of peptide loaded PBMCs as stimulators might be advantageous.
To further analyze the functionality of the activated hTERT-specific T cells we performed a cytotoxicity assay in patient F. In this assay it was assessed whether the hTERT-reactive T cells detected by measuring specific, intracellular IFN-production mediate cytotoxicity to hTERT+ target cells. However, the in vitro PBMC derived hTERT-specific T cells of this patient did not lyse the hTERT expressing target cells. This data might be related to the very low percentage of hTERT reactive T cells since the number of IFN-γ secreting T cells is closely correlated to their cytolytic activity (30). To detect very low numbers of cytolytic T cells analysis by Flow cytometry of cytolytic makers like granzyme and perforin could be performed in future studies.
Further functional analyses were prevented by the limited absolute number of T cells. After 15 days of in vitro stimulations the number of T cells dropped from 6×106 T cells to a median of 1.3×106 T cells. This drop was to some extent expected within the initial activation period. Most likely apoptosis of T cells not recognizing their specific antigen is the leading cause. However, also hTERT-specific T cells might have died within these 15 days due to exhaustion or culture conditions not facilitating survival of TAA-specific T cells. Allogeneic feeder cells like lymphoblastoid cell lines might be of better use for T cell expansion (22). These data indicate that for both clinical adoptive transfer studies as well as for further functional analyses the initial activation of hTERT-specific T cells need to be combined with efficient T cell expansion protocols (31).
These studies show, that by application of the standard cytokine approach hTERT-specific T cells can be generated in vitro in a subset of patients only. Although DCs in our study were phenotypically less matured by a TLR7/8-agonist in combination with a sCD40L, in PBMC samples of 2 patients the number of hTERT-specific T cells was improved. Other authors have previously shown the in vitro effectivity of this combination of a TLR7/8-agonist and the maturation signal sCD40L activating MART-1-specific CD8+ T cells with high functional avidity and enhanced tumor cell recognition (17). These findings were induced by high-levels of IL-12 expression. IL-12 produced by the TLR7/8-primed DC polarized CD4+ T cells for Th1 cytokine production leading to MART-1-specific CD8+ T cells (17). IL-12 being expressed by the TLR7/8-agonist and sCD40L primed DC greatly enhances T cell functional avidity (32). The analysis of the DC in our study was restricted to the expression of a maturation marker and costimulatory molecules. Only 50% of DCs that had been matured by the TLR7/8-agonist and sCD40L upregulated CD83 and CD86. Although CD80 was also upregulated in all DCs, this surface marker was expressed less intensely. However, the secretion of IL-12 is crucial for the induction of specific T-cell responses and may characterize the generated DC in more detail rather than expression of surface maturation molecules. In addition the adherent cells that we have designated DC after culture in IL-4 and GM-CSF could be further characterized by flow cytometry for specific DC markers like CD11c.
Further improvement of DC T cell stimulatory capacity might be achieved by the combination of cytokines with CD40- and TLR7/8 ligands. Paustian et al. (33) have shown that DC activated with IFN-gamma in combination with TLR4-, TLR7/8-agonists and CD40-ligands were capable of responding with strong IL-12 production. This quadruple activating cocktail was not tested for its capacity to sensitize T cells but further studies are needed to determine the best formulation for DC maturation. In a clinical phase I-study in patients with advanced malignant melanoma a subcutaneous vaccine consisting of melan-A-peptide26-35 in combination with the TLR9L CPG 7909 and montanide ISA-51 stronger activated CD8+ T cell responses than any other vaccine induced T cell-reaction in men (34).
Summarizing these data, even with the DC maturation protocols used in this study it was not possible to increase the number of immune responders. Only in PBMCs of a subgroup of lung cancer patients receiving standard platinum containing chemotherapy hTERT-specific T cells could be activated in vitro. Potentially these T cells can be isolated and expanded facilitating adoptive T cell transfer for treatment of tumor cells remaining after standard chemotherapy. In addition, hTERT-specific vaccinations might be simultaneously applied to first line chemotherapy protocols. The results of our study indicate, that following chemotherapy hTERT-specific T cell responses can be activated in vitro in a subgroup of NSCLC patients only. This activation by peptide pulsed cDC can be further increased by ligDC. We have used a set of hTERT peptides covering the MHC-class I molecules HLA A1, A2, A24 and the MHC-class II molecules HLA DR1/7/15. However, in our study the number of patients with these HLA-types was limited. Experimental settings using overlapping peptides covering the whole hTERT protein for T cell activation would potentially be advantageous. In such a study patients of may be all HLA-types could be included leading to high numbers of patients analyzed.
Naturally occurring T cells against multiple TAA are a common phenomenon in tumor patients. In lung cancer patients the number of TAA-reactive T cells was greatly different between the MAGE-A1, MAGE-A3 and hTERT peptides (27). Following these findings one could expect T cell reactivity to other TAAs in those patients who in the present study did not activate hTERT-specific T cell reactivity. Based on this assumption recent immunotherapy studies are investigating multiepitope peptides for T cell activation
We have not analyzed the frequency of base line T cell reactivity to hTERT. In a previous study the frequency of circulating hTERT-specific T cells was found significantly higher in newly diagnosed lung cancer patients as compared with healthy individuals (27) but varied widely. Upon diagnosis T cell responses to hTERT-peptides were detected in up to 40% of lung cancer patients only. Potentially in our study patients not activating hTERT-specific T cell in vitro did not have any base line hTERT-specific T cell reactivity.
The identification of appropriate immune surrogates of tumor-specific T-cell activation is still pending. A candidate predictive biomarker that identifies patients being able to activate hTERT-specific immune responses in vitro might be the presence of preexisting hTERT-specific T-cells. These tumor-specific T cells should have long term memory, lytic function, proliferative capacity, the ability to migrate to the tumor sites and could potentially be detected by advanced tetramer staining technologies.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329-60. [PubMed]
- Van Der Bruggen P, Zhang Y, Chaux P, et al. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev 2002;188:51-64. [PubMed]
- Bollard CM, Aguilar L, Straathof KC, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin’s disease. J Exp Med 2004;200:1623-33. [PubMed]
- Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005;23:2346-57. [PubMed]
- Van Tendeloo VF, Van de Velde A, Van Driessche A, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A 2010;107:13824-9. [PubMed]
- Meyerson M, Counter CM, Eaton EN, et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 1997;90:785-95. [PubMed]
- Kumaki F, Kawai T, Hiroi S, et al. Telomerase activity and expression of human telomerase RNA component and human telomerase reverse transcriptase in lung carcinomas. Hum Pathol 2001;32:188-95. [PubMed]
- Fujita Y, Fujikane T, Fujiuchi S, et al. The diagnostic and prognostic relevance of human telomerase reverse transcriptase mRNA expression detected in situ in patients with nonsmall cell lung carcinoma. Cancer 2003;98:1008-13. [PubMed]
- Gannagé M, Abel M, Michallet AS, et al. Ex vivo characterization of multiepitopic tumor-specific CD8 T cells in patients with chronic myeloid leukemia: implications for vaccine development and adoptive cellular immunotherapy. J Immunol 2005;174:8210-8. [PubMed]
- Vonderheide RH, Schultze JL, Anderson KS, et al. Equivalent induction of telomerase-specific cytotoxic T lymphocytes from tumor-bearing patients and healthy individuals. Cancer Res 2001;61:8366-70. [PubMed]
- Arai J, Yasukawa M, Ohminami H, et al. Identification of human telomerase reverse transcriptasederived peptides that induce HLA-A24-restricted antileukemia cytotoxic T lymphocytes. Blood 2001;97:2903-7. [PubMed]
- Domchek SM, Fox K, Recio A, et al. Immunological and clinical outcomes following telomerase peptide vaccination in patients with metastatic breast cancer. Proc. AACR 2006;4003a.
- Bernhardt SL, Gjertsen MK, Trachsel S, et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br J Cancer 2006;95:1474-82. [PubMed]
- Bolonaki I, Kotsakis A, Papadimitraki E, et al. Vaccination of patients with advanced non-small-cell lung cancer with an optimized cryptic human telomerase reverse transcriptase peptide. J Clin Oncol 2007;25:2727-34. [PubMed]
- Bendelac A, Medzhitov R. Adjuvants of immunity: harnessing innate immunity to promote adaptive immunity. J Exp Med 2002;195:F19-23. [PubMed]
- Xu S, Koldovsky U, Xu M, et al. High-avidity antitumor T-cell generation by toll receptor 8-primed, myeloid- derived dendritic cells is mediated by IL-12 production. Surgery 2006;140:170-8. [PubMed]
- Yao X, Ahmadzadeh M, Lu YC, et al. Levels of peripheral CD4(+)FoxP3(+)regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood 2012;119:5688-96. [PubMed]
- Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942-9. [PubMed]
- Asemissen AM, Keilholz U, Tenzer S, et al. Identification of a highly immunogenic HLA-A*01-binding T cell epitope of WT1. Clin Cancer Res 2006;12:7476-82. [PubMed]
- Nishikawa H, Jäger E, Ritter G, et al. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood 2005;106:1008-11. [PubMed]
- Gahn B, Siller-Lopez F, Pirooz AD, et al. Adenoviral gene transfer into dendritic cells efficiently amplifies the immune response to LMP2A antigen: a potential treatment strategy for Epstein-Barr virus--positive Hodgkin’s lymphoma. Int J Cancer 2001;93:706-13. [PubMed]
- Horton H, Russell N, Moore E, et al. Correlation between interferon- gamma secretion and cytotoxicity, in virus-specific memory T cells. J Infect Dis 2004;190:1692-6. [PubMed]
- Filaci G, Fravega M, Setti M, et al. Frequency of telomerase-specific CD8+ T lymphocytes in patients with cancer. Blood 2006;107:1505-12. [PubMed]
- Maecker B, von Bergwelt-Baildon MS, Anderson KS, et al. Rare naturally occurring immune responses to three epitopes from the widely expressed tumour antigens hTERT and CYP1B1 in multiple myeloma patients. Clin Exp Immunol 2005;141:558-62. [PubMed]
- Jonuleit H, Kühn U, Müller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol 1997;27:3135-42. [PubMed]
- Horn T, Grab J, Schusdziarra J, et al. Antitumor T cell responses in bladder cancer are directed against a limited set of antigens and are modulated by regulatory T cells and routine treatment approaches. Int J Cancer 2013. [Epub ahead of print]. [PubMed]
- Karanikas V, Zamanakou M, Soukou F, et al. Naturally occurring tumor-specific CD8+ T-cell precursors in individuals with and without cancer. Immunol Cell Biol 2010;88:575-85. [PubMed]
- Vonderheide RH, Anderson KS, Hahn WC, et al. Characterization of HLA-A3-restricted cytotoxic T lymphocytes reactive against the widely expressed tumor antigen telomerase. Clin Cancer Res 2001;7:3343-8. [PubMed]
- Scheibenbogen C, Lee KH, Mayer S, et al. A sensitive ELISPOT assay for detection of CD8+ T lymphocytes specific for HLA class I-binding peptide epitopes derived from influenza proteins in the blood of healthy donors and melanoma patients. Clin Cancer Res 1997;3:221-6. [PubMed]
- Kurokawa T, Oelke M, Mackensen A. Induction and clonal expansion of tumor-specific cytotoxic T lymphocytes from renal cell carcinoma patients after stimulation with autologous dendritic cells loaded with tumor cells. Int J Cancer 2001;91:749-56. [PubMed]
- Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol 1998;28:2760-9. [PubMed]
- Paustian C, Caspell R, Johnson T, et al. Effect of multiple activation stimuli on the generation of Th1-polarizing dendritic cells. Hum Immunol 2011;72:24-31. [PubMed]
- Speiser DE, Liénard D, Rufer N, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 2005;115:739-46. [PubMed]


