Development of a non-infectious rat model of acute exacerbation of idiopathic pulmonary fibrosis
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive interstitial lung disease with severe pulmonary fibrosis. The etiology of IPF remains unclear. Patients usually only survive 2–3 years after being diagnosed with IPF (1,2). The incidence and development of IPF are associated with epithelial cell injury, fibrocyte proliferation, inflammatory reactions, and extracellular matrix deposition (3,4). The pathological manifestation of IPF is usual interstitial pneumonia (UIP) (3,5). Currently, the mortality in patients with IPF is quite high. However, effective treatments for IPF are lacking (6). The main cause of IPF-associated death is acute exacerbation of IPF (AE-IPF), which accounts for more than 50% of IPF-related deaths (7). Kondoh et al. firstly described AE-IPF (8) and found that AE-IPF also presented diffuse alveolar damage (DAD) in addition to UIP (7). Most patients with AE-IPF are unable to tolerate bronchoscopy or lung biopsy because of their critical condition. Lack of the biopsy of AE-IPF substantially limits in-depth investigations of AE-IPF. Experimental animal models that could mimic AE-IPF would be useful tools to study AE-IPF.
Animal models of bleomycin (BLM)-induced IPF were initially described in 1970 (9). The rat model of IPF, which is developed by an intratracheal perfusion with BLM, has already been commonly used (10-12). Theoretically, a second intratracheal perfusion with BLM should induce additional lung injury in the rats that already develop pulmonary fibrosis from the first perfusion. The additional lung injury may resemble the pathological characteristics of AE-IPF. This study aims to test this approach to develop a rat model of AE-IPF. The pathological characteristics of the rat model were also investigated.
Methods
Chemicals and reagents
BLM was purchased from the Nippon Kayaku Co. Ltd. (Tokyo, Japan). Isoflurane and pentobarbital sodium were purchased from Yuyan Instruments Co., Ltd. (Shanghai, China). Neutral formalin (10%) was purchased from Wuhan Goodbio technology Co., Ltd. (Wuhan, China). Rat IL-6, IL-10, IL-17A and TGF-β enzyme-linked immunosorbent assay (ELISA) kits were purchased from Neo Bioscience Technology Co. (Shenzhen, China). Tissue RNA kit was purchased from Biomiga Inc. (San Diego, USA). The primer sequences were designed and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The real-time PCR (RT-PCR) kit was purchased from TOYOBO Life Science (Osaka, Japan).
Animals
The protocol for animal maintenance and experiments was approved by the Institutional Animal Care and Use Committee at Tongji University. A total of 90 male Sprague Dawley (SD) rats (SPF grade, body weight: 100±10 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd., China. The rats were maintained at 24±1 °C and a relative humidity of 40–80% according to the Guidelines for Animal Experimentation of Tongji University (Shanghai, China). The rats had free access to food and water and were housed for 1 week to adapt the new environment before being used for experiments.
Development of animal models
The rats were randomized into the following three groups: a BLM + BLM group (n=40), a BLM group (n=25), and a control group (n=25). BLM was dissolved in saline to a final concentration of 2.5×10-3 mg/L. All the rats were anesthetized by isoflurane inhalation and then intratracheally perfused with BLM (5 mg/kg) (for the BLM + BLM and BLM groups) or the same volume of saline (control group) by laryngoscopy (Penn-Century, Inc., LS-2-R, USA). The second perfusion was performed on day 28 after the first perfusion. Based on our preliminary experiments (see supplementary appendix, Figure S1), 7 mg/kg BLM was the optimal dose for the second perfusion in the BLM + BLM group. The same volume of saline was perfused in the other two groups (the BLM and control groups). Six rats were selected randomly from each group on day 31, day 35, and day 42 after the first perfusion, respectively, and then anesthetized by an intraperitoneal injection of 2% pentobarbital sodium. Subsequently, blood was collected from the abdominal aorta to analyze blood gas. After the rats were sacrificed by exsanguination, supernatant of the bronchoalveolar lavage fluid (BALF) from the right lung was preserved at −80 °C; the lung tissues were dissected. The left lung was preserved in liquid nitrogen for RT-PCR analysis. The anterior and middle lobe of the right lung was fixed in 10% neutral formalin for hematoxylin and eosin (H & E) staining and Masson staining, and the posterior lobe of the right lung was used to analyze lung water content (Figure 1).
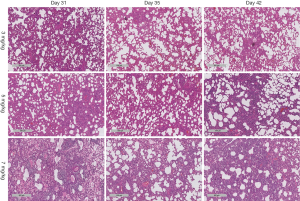

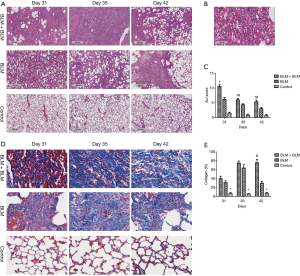
Histopathology of lung tissues
The anterior and middle lobe of the right lung was fixed in 10% neutral formalin for 48 hours, embedded in paraffin, and then sectioned for H & E staining and Masson staining. The stained lung tissue sections were analyzed on a pathological section scanner (4× magnification, Leica Biosystems, SCN400, Germany). All H & E-stained sections were analyzed and scored for acute lung injury (ALI) severity by two experienced pathologists, who were blinded for the group allocation (13). The average scores were used. ALI severity was scored based on the severity of the following four histopathological features: (I) alveolar congestion; (II) bleeding; (III) inflammatory cell infiltration; and (IV) alveolar wall thickening or a hyaline membrane formation. The severity of each feature was scored from 0–4 with 0 representing the least and 4 representing the most severe, and the total scores of the four features was used for statistical analysis. All Masson-stained sections were analyzed. Three observation fields were randomly selected from each Masson-stained section, and the percentage of blue areas, which represent collagen deposition, over the total tissue area (i.e., percentage area of collagen deposition) was calculated for each image using Image-Pro Plus 6.0 software.
Arterial blood gas analysis
Rats were anesthetized by an intraperitoneal injection of 2% pentobarbital sodium (2 mL/kg), and 1.5 mL blood from the abdominal aorta was then collected for immediate arterial blood gas analysis (Radiometer Medical ApS., ABL800, Denmark).
Lung water content
Lung water content was measure to estimate lung edema. A total of 250 mg lung lobe was collected from the same position of the lung from each rat and dried overnight in a freeze-dryer (Beijing Sihuan Company, LGJ-10D, China). The difference between dry weight and wet weight of each sample was calculated as lung water content (lung water content = wet weight − dry weight).
Measurement of inflammatory factors and albumin (ALB) in the BALF supernatant
The BALF was centrifuged (4 °C, 3,000 rpm, 5 minutes), and the supernatants were collected. The levels of inflammatory factors (IL-6, IL-10, IL-17A, and TGF-β) were analyzed using ELISA kits according to the manufacturer’s instruction. The absorbance at 450 nm was determined in a plate reader (Thermo Fisher Scientific Inc., Varioskan Flash, USA). ALB levels in the supernatant of BALF were measured using the bromocresol green combination method by the Laboratory Department of Shanghai Pulmonary Hospital.
Determination of NF-κB mRNA expression in lung tissues by RT-PCR
Total RNA was extracted from the lung tissues following the instruction from the kit (Biomiga, USA). cDNA was synthesized under the following condition: 65 °C for 5 minutes (heat denaturation) followed by immediate preservation on ice, 37 °C for 15 minutes, and 98 °C for 5 minutes. PCR was then performed according to the following condition: pre-denaturation at 95 °C for 1 minute, 40 cycles of denaturation at 95 °C for 15 seconds, annealing at 55 °C for 15 seconds, and extension at 60 °C for 40 seconds using a kit from TOYOBO Life Science. The linear correlation between the copy number of mRNA and cycle threshold (CT) was analyzed using Applied Biosystems® 7500 Fast Real-Time PCR Systems, and the standard curve was then prepared. Relative expression level of NF-κB mRNA was calculated according to the equation: 2−∆ct × 100%, ∆ct = CT of the target gene − CT of the internal reference (β-Actin). The primer sequences are described in Table 1.

Full table
Survival analysis and body weight measurement
Additional 18 rats in each group (n=18) were observed for survival analysis. Rat survival was monitor daily for 8 weeks after the first perfusion. Rat body weight was measured weekly.
Statistical analyses
The statistical analysis software Graphpad prism 5 (La Jolla, CA, USA) was used. Continuous variables are presented as mean ± standard deviation (SD). Inter-group comparisons were performed using the independent sample t-test and one-way AVOVA analysis of variance followed by Newman-Keuls multiple comparison test. Repeated measurement data were analyzed. Survival curve was plotted using the Kaplan-Meier method, and the survival time was compared by the log-rank test. P<0.05 represents statistical significance.
Results
Rats in the BLM + BLM group had worse pulmonary inflammation and fibrosis
H & E staining showed severe ALI in the BLM + BLM group. Normal alveolar structures disappeared; alveolar septa became markedly thickened; congestion and edema were developed; large amounts of inflammatory cells infiltrated into the interstitium and alveoli (Figure 2A). In addition, hyaline membranes were formed obviously on day 31 (Figure 2B). ALI score was significantly higher in the BLM + BLM group than in the BLM group (day 31: P=0.0005; day 35: P=0.0219; day 42: P=0.0118; Figure 2C). In the BLM + BLM group, the ALI score on day 31 was significantly higher than that on day 35 and day 42 (day 35: P=0.0002; day 42: P=0.0001; Figure 2C). Masson-staining showed large amounts of collagen deposition and apparent pulmonary fibrosis in the BLM + BLM and BLM groups (Figure 2D). Quantification of the Masson staining demonstrated significantly greater collagen deposition in the BLM + BLM and BLM groups than in the control group at all of the three time points (All P<0.05, Figure 2E). The collagen deposition was not statistically significantly different between the BLM + BLM group and the BLM group on day 31 and day 35, but was significantly higher in the BLM + BLM group than in the BLM group on day 42 (P=0.0007, Figure 2E).
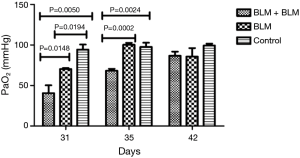
Arterial blood gas analysis
On day 3 and day 7 after the second perfusion (i.e., day 31 and day 35 after the first perfusion), the partial pressure of arterial oxygen (PaO2) was significantly lower in the BLM + BLM group than in the other two groups (day 31: BLM + BLM group vs. BLM group, P=0.0148, BLM + BLM group vs. control group, P=0.0050; day 35: BLM + BLM group vs. BLM group, P=0.0002, BLM + BLM group vs. control group, P=0.0024, Figure 3).
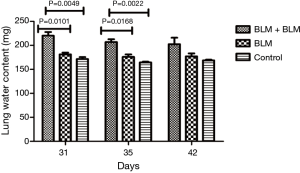
Lung water content
On day 3 and day 7 after the second perfusion (i.e., day 31 and day 35 after the first perfusion), the lung water content was significantly higher in the BLM + BLM group than in the other two groups (day 31: BLM + BLM group vs. BLM group, P=0.0101, BLM + BLM group vs. control group, P=0.0049; day 35: BLM + BLM group vs. BLM group, P=0.0168, BLM + BLM group vs. control group, P=0.0022, Figure 4).
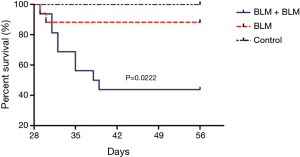
Survival analysis
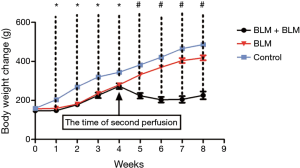
Rats in the BLM + BLM group were markedly less active and suffered respiratory embarrassment after the second BLM perfusion, and some rats developed thoracic deformity. On day 1 after the first perfusion, two rats in the BLM + BLM group and one in the BLM group died, and the control group had no death. On day 56, the mortality rate in the BLM + BLM group (56.25%, 9/16) was markedly higher than that in the BLM group (11.76%, 2/17, P=0.0222, Figure 5). One week after the first perfusion, rats receiving BLM perfusion had significantly lower body weight than rats in the control group (Figure 6). On day 56 (week 8), rats in the BLM + BLM group had significantly lower mean body weight (225.6±19.96 g) than rats in the BLM group (418.4±12.62 g, P<0.0001, Figure 6).
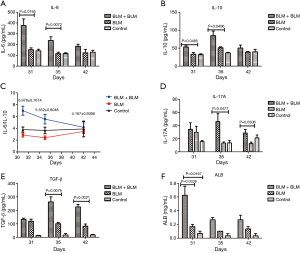
Rats in the BLM + BLM group had elevated levels of inflammatory factors and ALB in the BALF
During the first week after the second perfusion, IL-6 and IL-10 levels in the BALF in the BLM + BLM group were significantly higher than those in the other two groups (All P<0.05, Figure 7A,B), indicating that the second perfusion with BLM may induce acute inflammation. On day 31, the ratio of IL-6 to IL-10 in the BLM + BLM group was 6.9778±0.7674, and then the ratio gradually decreased at later time (Figure 7C). On day 35 and day 42, IL-17A level was evidently higher in the BLM + BLM group than in the BLM group (day 35, P=0.0477; day 42, P=0.0306, Figure 7D), and TGF-β level was markedly higher in the BLM + BLM group than in the BLM group (day 35, P=0.0076; day 42, P=0.0021, Figure 7E). These data suggest an exacerbation of pulmonary fibrosis after the second perfusion with BLM. On day 31, the ALB level was significantly higher in the BLM + BLM group than in the BLM and control groups (BLM + BLM group vs. BLM group, P=0.0338; BLM + BLM group vs. control group, P=0.0167, Figure 7F). These results are consistent with the ALI histopathology.
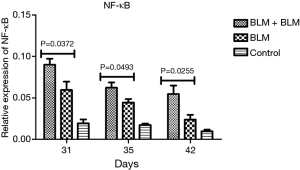
Rats in the BLM + BLM group had significantly increased expression of NF-κB mRNA in the lung tissue
RT-PCR revealed that on day 31, day 35, and day 42 after the first perfusion, the relative NF-κB mRNA level was significantly higher in the BLM + BLM group than in the BLM group (day 31, P=0.0372; day 35, P=0.0493; day 42, P=0.0255; Figure 8). NF-κB plays an important role in inflammatory response. An increased NF-κB expression suggests an elevated inflammatory response.
Discussion
Environmental pollution, seasonal changes, and lung-injuring drugs can induce AE-IPF (7,14). Patients with AE-IPF are usually in critical condition. Unfortunately, effective treatments for AE-IPF are still unavailable (15,16). Animal models to properly mimic AE-IPF are useful tools to study AE-IPF pathogenesis and develop effective therapies. In the current study, we developed a rat model of AE-IPF by two intratracheal perfusions with BLM.
An intratracheal perfusion with BLM induces acute alveolar inflammation accompanied with an increased infiltration of leukocytes, such as macrophages, granulocytes, and lymphocytes. These inflammatory cells stimulate fibroblast proliferation and myofibroblast activation, ultimately leading to pulmonary fibrosis (17). Animal models of BLM-induced pulmonary fibrosis have been widely used to study IPF (10-12). Brown et al. have shown that repetitive intratracheal perfusion with BLM worsens the BLM-induced lung injury and fibrosis in rats (18). Based on their study, we developed a rat AE-IPF model by a second intratracheal perfusion with BLM in rats that already had initial BLM-induced pulmonary fibrosis. We found that rats in the AE-IPF model group (BLM + BLM group) presented pulmonary fibrosis with apparent DAD and large amounts of hyaline membrane. In addition, rats receiving two perfusions with BLM had poor survival, elevated ALB levels in the BALF, increased lung water content, and high expression of various inflammatory factors. These findings suggest a successful development of a rat model of AE-IPF.
IL-6, a pro-inflammatory cytokine and an important biomarker for AE-IPF (19), is stimulated by NF-κB (20). IL-10, an anti-inflammatory cytokine, inhibits inflammation by suppressing NF-κB expression and activity (21). In the current study, IL-10 peaked (day 35) 4 days later than IL-6 (day 31) and the IL6/IL10 ratio reduced gradually in the BLM + BLM group, indicating that the second perfusion induces dynamic inflammatory changes in the lung. TGF-β has been found to be closely associated with pulmonary fibrosis (22). TGF-β and IL-6 can synergistically promote Th-17 T cell differentiation and stimulate IL-17A expression (23). IL-17A regulates neutrophil aggregation (24,25) and activates NF-κB to induce TNF-α production, thus causing inflammation and tissue injury (25,26). We found that on day 7 after the second perfusion with BLM, both TGF-β and IL-17A expression reached the maximum, suggesting a severe lung inflammation. The lung inflammation may eventually lead to chronic inflammation and fibrosis.
In the current study, the dynamic changes in the inflammatory factors, such as IL-6, IL-10, TGF-β, and IL-17A, indicate that the second perfusion may induce a rapid over-expression of pro-inflammatory factors, which counteract the anti-inflammatory factors. Thus, the net effects were acute inflammation and an exacerbation of the first perfusion-induced pulmonary fibrosis. These inflammatory events resemble the pathophysiological changes in patients developing AE-IPF.
Patients with AE-IPF often suffer severe infiltrative edema and alveolar collapse because of alveolar epithelial and endothelial cell damage, and consequently develop respiratory failure. PaO2 reduction is one of the key criteria to diagnose AE-IPF (15,27). Rats in the BLM + BLM group presented a significant increase in lung water content and a sharp decrease in PaO2 1 week after the second perfusion. Furthermore, rats in the AE-IPF model group (BLM + BLM group) also had respiratory embarrassment and high mortality rate. These pathophysiological features are similar to the clinical manifestation of patients with AE-IPF.
Our results showed that a second perfusion with BLM (7 mg/kg) induced apparent DAD in the rats that already developed pulmonary fibrosis induced by the first perfusion. The worst lung injury occurred on day 3 after the second perfusion, and the ALI gradually exacerbated the pulmonary fibrosis. The course of lung injury development in rats receiving two perfusions is very similar to that in patients with AE-IPF. The previous study has demonstrated a murine model of gammaherpesvirus-induced pulmonary fibrosis (28). However, the roles of infection in the development of AE-IPF in patients are still unclear. The rat model of AE-IPF in the current study is simpler and safer than the animal models of AE-IPF induced by infection, and shows satisfactory reproducibility. Previous studies that used repetitive intratracheal perfusion with BLM have shown that consecutive weekly intratracheal instillations of BLM result in severe pulmonary fibrosis (18,29). In the rat model of the current study, the ALI induced by the second perfusion was associated with lung fibrosis, and this fibrosis-related ALI mimics human AE-IPF. In our previous study, we have successfully established a mouse model of AE-IPF by twice BLM administrations (30). The current study has novelty although we have already published a mouse model of AE-IPF using two administrations of BLM. First, we tested dose response and time course in the rat model in the current study (supplementary appendix file, Figure S2). Second, compared to the mouse model, the rat model was established by intratracheal perfusion with BLM or saline under laryngoscopy, which substantially improves the efficiency of intratracheal perfusion with BLM and reduces the risk that the perfusion solution may mistakenly enter the esophagus. Third, PaO2 reduction is one of the key criteria to diagnose AE-IPF. Thus, we added arterial blood gas analysis in the current study for a more accurate assessment of AE-IPF in the rat model. The last but not the least, most of the patients with AE-IPF are usually so critical ill that oral medication may fail to relieve their symptoms efficiently. Therefore, medications are usually administered via intravenous injection for those patients. It is critical to develop an experimental animal model of AE-IPF, which can be used to test drugs administered by intravenous injection. Compared with mice, rats have thicker caudal vein and thus intravenous injection is technically easier for rats than mice. Thus, this rat model of AE-IPF may be more useful to test new drugs than the mouse model.

Conclusions
The pathophysiological characteristics of rats receiving two perfusions with BLM resemble to those of patients with AE-IPF. Thus, a second perfusion with BLM appears to induce AE-IPF and may be used to model AE-IPF in rats.
Acknowledgements
The authors thank Professor Robert P. Baughman for proofreading the manuscript.
Funding: This study was funded by grants from the National Science Foundation of China (No. 91442103, 81500052, and 81570057), Ministry of Science and Technology of the People’s Republic of China (2016YFC1100200, 2016YFC1100204), the Health Bureau Program of Shanghai Municipality (SHDC12014120 and 2013SY047), and the Science Foundation Tongji University (No. 1511219020).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee of Shanghai Pulmonary Hospital (No. 2014fk04).
Supplementary methods
Rats were randomized into three groups: a low-dose group (3 mg/kg, LD group, n=20), a moderate-dose group (5 mg/kg, MD group, n=20), and a high-dose group (7 mg/kg, HD group, n=20). Bleomycin (BLM) was dissolved in saline to reach the final concentration of 2.5 mg/mL. All of the rats were anesthetized by isoflurane inhalation and then intratracheally perfused with 5 mg/kg BLM by laryngoscopy. The second perfusion with BLM at 3, 5, or 7 mg/kg was performed on day 28 after the first perfusion. Three rats were selected randomly from the three dose groups on day 31, day 35, and day 42 after the first perfusion, respectively, and then anesthetized by an intraperitoneal injection of 2% pentobarbital sodium. The rats were subsequently sacrificed by exsanguination.
Supernatant of the bronchoalveolar lavage fluid (BALF) from the right lung was collected to analyze the level of inflammatory factors (IL-6, IL-10 and TGF-β) using an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions. The left lung lobe was fixed in 10% neutral formalin for 48 hours, embedded in paraffin, and sectioned for hematoxylin and eosin (H & E) staining. Histology sections were analyzed on a pathological section scanner. All H & E-stained sections were analyzed with LEICA SCN400 (20×, scale bars represent 500 µm) by two experienced pathologists, who were blinded for the group allocation.
References
- Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684-91. [Crossref]
- Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;157:199-203. [Crossref]
- Harari S, Caminati A. IPF: new insight on pathogenesis and treatment. Allergy 2010;65:537-53. [Crossref]
- Daccord C, Maher TM. Recent advances in understanding idiopathic pulmonary fibrosis. F1000Res 2016;5. [Crossref]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref]
- Raghu G, Rochwerg B, Zhang Y, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 2015;192:e3-19. [Crossref]
- Ryerson CJ, Cottin V, Brown KK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: shifting the paradigm. Eur Respir J 2015;46:512-20. [Crossref]
- Kondoh Y, Taniguchi H, Kawabata Y, et al. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest 1993;103:1808-12. [Crossref]
- Okamoto T, Amano T, Wada T, et al. Experimental pulmonary fibrosis induced by bleomycin. Igaku To Seibutsugaku 1970;80:299-301.
- Wang C, Song X, Li Y, Han F, et al. Low-dose paclitaxel ameliorates pulmonary fibrosis by suppressing TGF-β1/Smad3 pathway via miR-140 upregulation. PLoS One 2013;8:e70725. [Crossref]
- Gao L, Tang H, He H, et al. Glycyrrhizic acid alleviates bleomycin-induced pulmonary fibrosis in rats. Front Pharmacol 2015;6:215. [Crossref]
- Wang WJ, Liao B, Zeng M, et al. The effects of aerosolized STAT1 antisense oligodeoxynucleotides on rat pulmonary fibrosis. Cell Mol Immunol 2009;6:51-9. [Crossref]
- Mikawa K, Nishina K, Takao Y, et al. ONO-1714, a nitric oxide synthase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits. Anesth Analg 2003;97:1751-5. [Crossref]
- Donahoe M, Valentine VG, Chien N, et al. Autoantibody-Targeted Treatments for Acute Exacerbations of Idiopathic Pulmonary Fibrosis. PLoS One 2015;10:e0127771. [Crossref]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref]
- Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356-63. [Crossref]
- Mouratis MA, Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med 2011;17:355-61. [Crossref]
- Brown RF, Drawbaugh RB, Marrs TC. An investigation of possible models for the production of progressive pulmonary fibrosis in the rat. The effects of repeated intratracheal instillation of bleomycin. Toxicology 1988;51:101-10. [Crossref]
- Collard HR, Calfee CS, Wolters PJ, et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2010;299:L3-7. [Crossref]
- Liu W, Jiang HL, Cai LL, et al. Tanreqing Injection Attenuates Lipopolysaccharide-Induced Airway Inflammation through MAPK/NF-κB Signaling Pathways in Rats Model. Evid Based Complement Alternat Med 2016;2016:5292346.
- Lutay N, Håkansson G, Alaridah N, et al. Mycobacteria bypass mucosal NF-kB signalling to induce an epithelial anti-inflammatory IL-22 and IL-10 response. PLoS One 2014;9:e86466. [Crossref]
- Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc 2012;9:111-6. [Crossref]
- Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007;8:967-74. [Crossref]
- Li L, Huang L, Vergis AL, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 2010;120:331-42. [Crossref]
- Bhat MA, Al-Omar MA, Ansari MA, et al. Design and Synthesis of N-Arylphthalimides as Inhibitors of Glucocorticoid-Induced TNF Receptor-Related Protein, Proinflammatory Mediators, and Cytokines in Carrageenan-Induced Lung Inflammation. J Med Chem 2015;58:8850-67. [Crossref]
- Mori K, Fujisawa T, Kusagaya H, et al. Synergistic Proinflammatory Responses by IL-17A and Toll-Like Receptor 3 in Human Airway Epithelial Cells. PLoS One 2015;10:e0139491. [Crossref]
- Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636-43. [Crossref]
- McMillan TR, Moore BB, Weinberg JB, et al. Exacerbation of established pulmonary fibrosis in a murine model by gammaherpesvirus. Am J Respir Crit Care Med 2008;177:771-80. [Crossref]
- Wild JS, Hyde DM, Giri SN. Dose and regimen effects of poly ICLC, an interferon inducer, in a multi-dose bleomycin model of interstitial pulmonary fibrosis. Pharmacol Toxicol 1994;75:42-8. [Crossref]
- Wei YR, Qiu H, Wu Q, et al. Establishment of the mouse model of acute exacerbation of idiopathic pulmonary fibrosis. Exp Lung Res 2016;42:75-86. [Crossref]


