Current status of spray cryotherapy for airway disease
Introduction
Extreme cooling for a therapeutic effect or cryotherapy is employed for different indications including ablation of benign and malignant neoplasms, ablation of granulation tissue, treatment of infections, nerve ablation for chronic pain, bronchoscopic biopsies, and even cosmetic surgery (1-6). The cooling temperature can range significantly from 0 °C to almost –200 °C. The most common modes of application of cryotherapy are by direct contact with a probe [cryoprobe therapy (CPT)] or by catheter-based delivery or spraying of a liquid cryogen [cryospray therapy (CST)]. The effect on tissues can therefore also differ depending on the temperature and modality applied.
Direct contact cryoprobes are cooled using the Joule-Thompson effect. A compressed gas such as nitrous oxide is suddenly released into a closed tip causing a drop of temperature of this probe and can achieve cooling temperatures of down to –90 °C. Directly adjacent tissues will become cooler and eventually freeze over a period of time, usually minutes. This causes freezing of extracellular water and actual dehydration of cells.
CST on the other hand relies on rapidly releasing a liquid with a low boiling point (BP) through a catheter in order to induce a hypothermic effect on the tissues. The most commonly used cryogens for CST are nitrogen and oxygen. Liquid nitrogen spray creates a non-contact cryogenic effect on tissues. The large difference in temperature between the BP of this liquid (–196 °C) and normal body temperature (37 °C) leads to a rapid flash freezing of tissue. This leads to intracellular ice crystal formation and aggregation with eventual rupture of intracellular organelles and cell death. Immediately upon release from the catheter, the liquid boils and evaporates, rapidly increasing in volume. It is therefore also essential to evacuate or “vent” this gas in order to avoid complications of pressure buildup or barotrauma.
Brief history of cryotherapy
The origins of cryotherapy can first be found in the literature dating back to 1850 when Arnott et al. first published the benefits of local application of a cooling solution used to treat pain. This initial solution was cooled by a mixture of a salt and crushed ice (1). The first use of liquid nitrogen in medicine was described by Allington in 1950 for treatment of dermatologic lesions (2). The liquid was applied by direct swabbing on the skin. Cooper and Lee introduced a cryosurgical probe in 1961 which was cooled by liquid nitrogen traveling down a hollow probe (3,4). This was used to directly ablate areas in the cerebral cortex for treatment of neurologic diseases such as Parkinsonism. The probe was inserted through a small craniotomy and local anesthesia in order to allow examination of the patient during the procedure (5).
The first successful delivery system of a cryoprobe into the airway was by Grana et al. using a direct contact cryoprobe which was advanced through a rigid bronchoscope for ablation of “unwanted tissue”. In 1969 they described their technique in an animal model (7). In 1981, Sanderson et al. also described their experience with cryoprobe ablation using bronchoscopy and a nitrogen cooled cryoprobe in a series of patients with lesions that were deemed non-surgical. The procedure had a 3% operative mortality rate (3,8).
The first description of spray cryotherapy (SCT) was initially described using liquid nitrogen spray in 1997 by Pasricha et al. in a canine esophageal model (9). Later in 1999 Johnston et al. also described the endoscopic SCT system in a porcine model to examine its effects on the esophagus. They used the term “cryoburn” for the treatment lesion, which was defined as the visualization of the white frost on tissues after initiation of the spray (10). In 2005, Johnston et al. then described the use of SCT clinically in humans for ablation of high-grade dysplasia in Barrett’s esophagus. At the time of their 2005 pilot study, the only other endoscopic ablative method that was FDA approved for the treatment of high-grade dysplasia in Barrett’s esophagus was photodynamic therapy. The authors were able to show in a series of 11 patients that the use of SCT for the ablation of high-grade dysplasia in Barrett’s esophagus was efficacious, safe and easy to perform (11). This study paved the way for FDA approval for the first-generation device (TruFreeze system, CSA Medical Inc., USA) in 2007. Two subsequent studies by Greenwald et al. and Shaheen et al. using this same device showed that liquid nitrogen CST was able to eradicate 97% of cases of high grade dysplasia in Barrett’s esophagus and 72% of T1 esophageal cancers (12,13).
Au et al. showed in an animal study that SCT was feasible for intraluminal application to endobronchial tissue. They were able to reliably reproduce the effects of the SCT in order to cause superficial necrosis extending from the mucosa to the cartilage in the airway (14). Due to concerns about barotrauma, it was slower to adapt this technology in the airway. Krimsky et al. published a case series in 2010 where they used SCT for the treatment of three patients with glottic and subglottic stenosis which resulted in patency of the stenosed areas with some degree of normalization of the mucosa (15). In 2011 and 2012, Fernando and Finley described two multicenter studies using SCT with the first generation device in the treatment of benign and malignant airway disease.
The current commercially available device for liquid nitrogen delivery (G2 TruFreeze system, CSA Medical Inc., USA) shown in Figure 1 has been modified from the first generation device by providing two adjustable flow rates at 25 watts (normal flow) and 12.5 watts (low flow). The current device received FDA approval for the destruction of unwanted tissue in 2012. The low flow rate may allow for slower delivery and slower buildup of pressure in case of inadequate venting. The first report of SCT for central airway disease using the new adjustable flow device was by Browning et al. in 2013 who reported a case series of four patients with malignant airway disease. It showed the feasibility of this treatment and some of its unique advantages such as in the presence of stents and its use to control bleeding (16).

Mechanism of action
The mechanism of tumor ablation by cryotherapy is based on the freezing of extra and intracellular tissue water causing formation of ice crystals. Slower cooling such as by direct contact tends to cause mainly extracellular ice which leads to cell death by dehydration and electrolyte imbalance. SCT of liquid nitrogen on the other hand leads to very rapid cooling with intracellular ice crystallization and subsequent cell death by direct destruction of intracellular organelles (3).
Benign strictures, however, are acellular and the mechanism of action is therefore different. Dermatology literature has shown that cryotherapy can cause remodeling of the connective tissue matrix which subsequently may allow for a more easily dilated stricture (17,18). Kim et al. showed that the use of cryotherapy in benign bronchial lesions allowed for easier dilation of a softer scar which may help prevent the common complication of bronchial laceration during balloon dilation which may cause further scarring and stricture formation (19).
Studies have shown that with SCT, there is relative sparing of the extracellular matrix in tissues (3). This matrix can then form a scaffold for subsequent tissue healing without a severe fibrotic response (18,20) and thus less long-term scarring (3,19). It has also been shown that although the matrix remains intact, SCT has an immediate softening effect on the fibrotic stricture allowing easier and wider dilation with less chance of laceration. This may be due to a remodeling of the connective tissue (20,21).
Technique of SCT
The truFreeze System (CSA Medical, Inc., Baltimore, MD, USA) is used to spray liquid nitrogen through a 7F catheter that is passed into an endoscope. In the esophagus, a sump tube is advanced into the stomach and attached to suction to perform active venting of the gas. However, in the airway, this cannot be performed as there is not enough room to place both a bronchoscope and a suction catheter in the airway while still allowing for ventilation of the patient. In addition, any active suctioning would deplete the lungs of air and cause severe atelectasis. The concept of passive venting is therefore, employed in the airway. It relies on the fact that gas will egress through the path of least resistance. As long as there is a patent and unobstructed opening of sufficient diameter, gas under pressure will vent through it. Ensuring adequate venting of the excess nitrogen gas to prevent barotrauma is essential during the procedure.
Protocol for passive airway venting
- Deflate the endotracheal tube cuff;
- Disconnect the tube from the ventilator circuit;
- Visualize adequate passive egress (misting) of gas through the endotracheal tube or rigid bronchoscope;
- Confirm the absence of chest wall rise during the sprays;
- Remove bronchoscope between treatments;
- Closely monitor the heart rate, blood pressure, oxygenation and EKG tracings;
- When treating multiple strictures, the more proximal lesion is treated first in order to allow adequate proximal venting when treating the distal lesion;
- If at any time there is concern that passive venting is compromised, SCT is immediately aborted and the bronchoscope is immediately removed.
The use of this protocol is essential to help prevent the potentially catastrophic complications of tension pneumothorax, pneumomediastinum and nitrogen gas embolism.
The procedure is done under general anesthesia with a large bore endotracheal tube (>8 mm) or rigid bronchoscope. Glottic or subglottic lesions can be treated through a laryngeal mask airway (LMA) or suspension laryngoscopy although this is not recommended in more distal lesions where venting may be compromised.
The spray is applied in intervals of 5 seconds, timed from when visible frost formation has covered at least 50% of the target area. Complete thawing for at least 30 seconds is allowed after each application.
As mentioned previously, the current device can generate two flow rates; normal flow and low flow. Whereas the low flow rate may allow for slower delivery and buildup of pressure in case of inadequate venting, it also requires longer periods of apnea while administering the treatments. In the authors experience it is preferable to use the normal flow rate once adequate venting is confirmed in order to avoid potential hypoxia from the longer treatment times.
The number of cycles of spray and thawing is based on disease burden and response. Usually, four cycles are administered followed by dilation and/or debridement then a final two cycles.
There is usually no immediately visible effect after CST. For this reason, all patients undergo an additional procedure to SCT including balloon dilation, stent placement, and/or mechanical debridement depending on the nature of the lesion.
Adjunctive modalities
While CST does show promise in treating both malignant and benign pathology of the central airways, it is not a sole treatment modality. It appears to be most successful when used in conjunction with mechanical dilation methods such as balloon dilation, mechanical debridement of tissue and stent placement. Browning et al. utilized adjunct modalities in 39% of their procedures which included mechanical debulking, electrocautery or argon beam coagulation, laser, cryoprobe and stent placement (22). Fernando et al. used the balloon dilation universally in their treatment of non-malignant strictures of the airway (20).
The safe use of the CST in the vicinity of stent placement, covered or uncovered, is an important benefit of this modality. Many of the patients that are being treated have had prior intervention and attempts at maintaining their airway. In our experience, we had a number of prior lung transplant patients who were treated for anastomotic complications with stents. This is becoming a more common technique and is particularly important at transplant centers. Dutau et al. showed in a single institution, retrospective study that the use of stents to treat lung transplant associated airway complications was both feasible and provided significant improvement in patient symptoms and outcomes (23). In our experience, we utilize adjunct treatments universally and have found no ill effects of such practice.
Advantages of SCT
SCT is technically quite simple and can be performed as an outpatient procedure, similar to any other routine bronchoscopy. The therapy compared to thermal or mechanical modalities is painless and may actually have an analgesic effect on nerve fibers. In addition, due to its vasoconstrictive effect, it is beneficial in achieving hemostasis on bleeding granulation tissue or tumors.
Since there is no heat delivery, there is also no combustion risk. It is therefore possible to administer high concentration oxygen during treatments and it is therefore ideal when performing ablations on patients who require high oxygen delivery. For the same reason, it can be used in cases with indwelling tracheobronchial stents, as opposed to other thermal modalities which can cause damage or combustion of stent material
In CPT, the tissue is cooled in a radial fashion from the area of contact. In contrast, SCT allows a more even and linear distribution of the hypothermic effect over a larger area with a more uniform effect on the entire treated lesion
Risks of SCT
Despite these advantages and the relative simplicity of the procedure, there is also a risk for severe barotrauma if the gas is not vented. When liquid nitrogen is released into the warm airway, there is almost instantaneous conversion to gas with resultant volume expansion on the order of 1:645. The main risks are therefore those of unrecognized barotrauma and although rare, can be serious and fatal. They include pneumothorax, pneumomediastinum, and nitrogen gas embolism. Other risks are those associated with the necessary apnea while holding ventilation during the treatments including hypoxia, hypercarbia, respiratory acidosis and bradycardia. Table 1 compares the complications of different studies in using this modality.
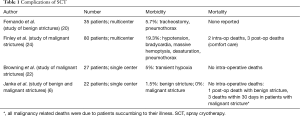
Full table
Indications and contraindications of SCT
The indications for CST in the airway are mainly stenotic lesions, whether benign or malignant. There have also been reports of its benefit in treatment of cough associated with malignant disease and chronic bronchitis (22).
Contraindications for SCT however include all situations where venting may be inadequate or where there is a potential for escape of nitrogen gas outside the airway and into the body. Venting may be compromised when treating distal lesions due to the relatively large diameter of the bronchoscope in relation to the smaller airway. In general; it is not recommended to treat strictures distal to the bronchus intermedius or left main-stem bronchus
In addition, extremely tight strictures (>90%) may cause gas to become trapped distally. It is safer to dilate such strictures prior to SCT.
Transmural bronchial invasion by tumor is a situation which may subsequently lead to fistula formation after cryospray ablation of the tumor. If there is obvious destruction of the wall of the airway and invasion by an extrinsic tumor, it may be safer not to treat such lesions with this modality. Of course, a stricture in the presence of an established bronchial fistula or anastomotic dehiscence should not be treated with SCT which may cause pneumomediastinum or pneumothorax in these situations.
Outcomes of SCT
In contrast to its use in esophageal disease, the published literature on the use of SCT in the treatment of airway diseases is still quite limited. In order to grade the degree of stenosis, we used the system described by Finley and Fernando which grouped stenoses into four quartiles; <25%, 26–50%, 51–75% and >75% and each quartile was given a score of 1–4.
Fernando et al. first published a multi-institutional study of the feasibility of CST and balloon dilation for benign strictures of the airway in 2011. They showed a statistically significant improvement of stricture diameter from 3.5 pre-treatment to 2.03 post-treatment (20). They also showed a complication rate of 5.7% with no reported mortalities in their series (18,20). Their complications included tracheostomy in a patient who developed glottis edema and pneumothorax which was thought to have developed due to treatment of a distal lesion prior to treating a more proximal one. Finley et al. followed with a similar study on a series of 80 patients treated with SCT for malignant airway disease in 2012. In this series, pretreatment airway occlusion was graded as more than 75% in 74% of patients, but only eight patients had more than 75% narrowing after treatment (24). They reported a 19.3% complication rate which included two intraoperative deaths. There was one emergent chest tube placed for a pneumothorax, one airway tear during mechanical debridement treated without intervention, one episode of massive hemoptysis treated with SCT, and one cardiac arrest (24). The two intraoperative deaths both had similar events which consisted of bradycardia, ST segment changes, hypoxemia and PEA arrest. There were also three postoperative deaths which included patients who were transitioned to comfort care.
Browning et al. described an institutional experience treating malignant airway disease with SCT in a recent publication in 2015. They quoted a 5% complication rate which included only episodes of transient hypoxia that resolved and did not lead to any measurable morbidity or mortality (22).
We have recently completed a single institution study to review our own experience with SCT (6). Our study included 22 patients, who underwent 66 bronchoscopies with 87 lesion-treatments. The patients were predominantly male (64%), with a median age of 61.5 [28–75] years. The cause of airway stenosis was benign in 10 (45.5%) and malignant in 12 (54.5%) patients. The majority of the benign lesions were anastomotic strictures for lung transplant while most of the malignant lesions were secondary to primary lung cancer.
At initial bronchoscopic evaluation, the median grade of stenosis was 4 for malignant disease and 3.5 for benign disease. The median final post-treatment grade of stenosis was 2 for malignant and 1 for benign disease. The median improvement in grade of stenosis after treatment was 2 for both malignant and benign causes (Wilcoxon test P=0.92). Final patency of Grade 1 was achieved in 42% of malignant stenosis and 80% of benign. Overall, 86.4% of patients had an improvement in grade of stenosis after treatment. Table 2 shows our results and compares our outcomes between patients with benign and malignant disease. Patients with benign disease generally required four treatments over 6–8 weeks, while those with malignant disease required 3–4 treatments over 2–3 weeks, in order to achieve adequate patency. Benign disease appears to require more treatments in order to achieve the same results as malignant tumors. This is likely based on the cellular and structural differences between the benign and malignant diseases.
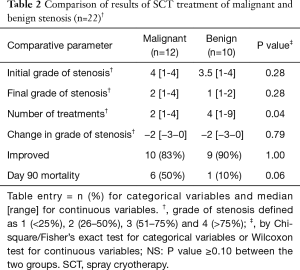
Full table
In our study, there were no intraoperative deaths. In the benign group, there was one death (10%) within 90 days in a patient with preexisting ventilator dependent respiratory failure 3 months after double lung transplant. Ninety day mortality in the malignant group was 50%, with all deaths related to progression of disease. The rate of procedure-related morbidity was 1.5% (1 complication in 66 procedures performed). The single complication occurred in a lung transplant patient, who was 7 months post-transplant and had an anastomotic stenosis in addition to a pre-existing, small anastomotic dehiscence. This patient required re-intubation for pulmonary edema prior to eventual recovery and discharge.
We believe that these results support the use of SCT in both benign and malignant airway strictures. They also highlight the need for appropriate protocols as defined above in order to prevent, mitigate, and treat the potential complications as well as provide appropriate informed consent for the prospective patients who are to undergo this new treatment. We also believe that an established diagnosis of airway perforation or dehiscence is a contraindication to SCT.
Areas for research
SCT is a relatively new procedure in the field of endobronchial ablative therapies. Published studies have helped define feasibility and safety for the use of the SCT delivery system in the airway and have outlined potential protocols to be followed. However, all the studies on this topic have been retrospective case reports and case series. There is therefore a need for both clinical and basic science research. Since the major risk of this procedure is barotrauma, animal research is necessary in determining the correlation of treatment to airway pressures and gas content. Also, little is known about the cellular and subcellular effects of this modality on tissues. More research is therefore needed on the molecular level in order to fully understand the effects of extreme hypothermia have on malignant tumors, benign strictures and normal tissues. Additionally, it would be interesting to determine whether there are any systemic effects, e.g., immune, from the ablation of tumors in these patients.
More information is also necessary on determining the best protocol in different situations, e.g., optimum duration of treatment cycle and number of cycles. In addition it is important to determine the need and timing of follow-up endoscopic evaluation and re-treatment.
Ultimately, a randomized controlled study comparing SCT to other endoscopic treatment such as cryoprobe, laser or argon plasma coagulation would help determine the best approach to bronchial lesions in an arena with numerous available modalities but no clear guidelines as to which is the best approach.
Summary
Patients with endobronchial or endotracheal strictures have been shown to benefit from CST. It can be safely performed as an outpatient procedure by flexible bronchoscopy to most targets in the central airway.
Surgeons with experience in the delivery system have developed their own protocols based on their individual experience. Our own experience has shown that the use of the SCT delivery system is effective and feasible for both benign and malignant strictures of the airway with an approximately 2 grade improvement in the degree of stenosis according to the previously described system.
As we continue to expand our knowledge, it is of utmost importance to contribute our experience with this technology to the literature. Despite its many potential advantages, it continues to be an area in need of scientific validation. Such validation may allow it to become more widely available, ultimately leading to improvement in patient quality of life, outcomes and life expectancy. What was once a limiting disease that greatly affected the way of life for those affected can now be treated on an outpatient basis many times over with minimal morbidity and mortality.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Arnott J. Practical illustrations of the remedial efficacy of a very low or anæsthetic temperature.—I. In cancer. The Lancet 1850;56:257-59. [Crossref]
- Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med 1950;72:153-5. [PubMed]
- Yiu WK, Basco MT, Aruny JE, et al. Cryosurgery: A review. Int J Angiol 2007;16:1-6. [Crossref] [PubMed]
- Cooper IS, Lee AS. Cryostatic congelation: a system for producing a limited, controlled region of cooling or freezing of biologic tissues. J Nerv Ment Dis 1961;133:259-63. [Crossref] [PubMed]
- Copper IS. Cryogenic surgery: a new method of destruction or extirpation of benign or malignant tissues. N Engl J Med 1963;268:743-9. [Crossref] [PubMed]
- Janke KJ, Abbas AE, Ambur V, et al. The Application of Liquid Nitrogen Spray Cryotherapy in Treatment of Bronchial Stenosis. Innovations (Phila) 2016;11:349-54. [Crossref] [PubMed]
- Grana L, Kidd J, Swenson O. Cryogenic techniques within the tracheobronchial tree. Journal of Cryosurgery 1969;2:62-7.
- Sanderson DR, Neel HB 3rd, Fontana RS. Bronchoscopic cryotherapy. Ann Otol Rhinol Laryngol 1981;90:354-8. [Crossref] [PubMed]
- Pasricha PJ, Hill S, Wadwa KS, et al. Endoscopic cryotherapy: experimental results and first clinical use. Gastrointest Endosc 1999;49:627-31. [Crossref] [PubMed]
- Johnston CM, Schoenfeld LP, Mysore JV, et al. Endoscopic spray cryotherapy: a new technique for mucosal ablation in the esophagus. Gastrointest Endosc 1999;50:86-92. [Crossref] [PubMed]
- Johnston MH, Eastone JA, Horwhat JD, et al. Cryoablation of Barrett's esophagus: a pilot study. Gastrointest Endosc 2005;62:842-8. [Crossref] [PubMed]
- Greenwald BD, Dumot JA, Abrams JA, et al. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc 2010;71:686-93. [Crossref] [PubMed]
- Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 2010;71:680-5. [Crossref] [PubMed]
- Au JT, Carson J, Monette S, et al. Spray cryotherapy is effective for bronchoscopic, endoscopic and open ablation of thoracic tissues. Interact Cardiovasc Thorac Surg 2012;15:580-4. [Crossref] [PubMed]
- Krimsky WS, Rodrigues MP, Malayaman N, et al. Spray cryotherapy for the treatment of glottic and subglottic stenosis. Laryngoscope 2010;120:473-7. [Crossref] [PubMed]
- Browning R, Parrish S, Sarkar S, et al. First report of a novel liquid nitrogen adjustable flow spray cryotherapy (SCT) device in the bronchoscopic treatment of disease of the central tracheo-bronchial airways. J Thorac Dis 2013;5:E103-6. [PubMed]
- Li AK, Ehrlich HP, Trelstad RL, et al. Differences in healing of skin wounds caused by burn and freeze injuries. Ann Surg 1980;191:244-8. [Crossref] [PubMed]
- Fernando HC, Sherwood JT, Krimsky W. Endoscopic therapies and stents for benign airway disorders: where are we, and where are we heading? Ann Thorac Surg 2010;89:S2183-7. [Crossref] [PubMed]
- Kim JH, Shin JH, Song HY, et al. Tracheobronchial laceration after balloon dilation for benign strictures: incidence and clinical significance. Chest 2007;131:1114-7. [Crossref] [PubMed]
- Fernando HC, Dekeratry D, Downie G, et al. Feasibility of spray cryotherapy and balloon dilation for non-malignant strictures of the airway. Eur J Cardiothorac Surg 2011;40:1177-80. [PubMed]
- Shepherd JP, Dawber RP. Wound healing and scarring after cryosurgery. Cryobiology 1984;21:157-69. [Crossref] [PubMed]
- Browning R, Turner JF Jr, Parrish S. Spray cryotherapy (SCT): institutional evolution of techniques and clinical practice from early experience in the treatment of malignant airway disease. J Thorac Dis 2015;7:S405-14. [PubMed]
- Dutau H, Cavailles A, Sakr L, et al. A retrospective study of silicone stent placement for management of anastomotic airway complications in lung transplant recipients: short- and long-term outcomes. J Heart Lung Transplant 2010;29:658-64. [Crossref] [PubMed]
- Finley DJ, Dycoco J, Sarkar S, et al. Airway spray cryotherapy: initial outcomes from a multiinstitutional registry. Ann Thorac Surg 2012;94:199-203; discussion 203-4. [Crossref] [PubMed]


