Results of neoadjuvant therapy followed by esophagectomy for patients with locally advanced thoracic esophageal squamous cell carcinoma
Introduction
For patients diagnosed with locally advanced esophageal cancer, neoadjuvant therapy followed by surgery is the most common approach (1). However, randomized trials did not result in consistent conclusions (2-7). The strategy remains investigational, especially for patients with esophageal squamous cell carcinoma (ESCC), which is the predominant type in China (8). We retrospectively reviewed all of the patients underwent surgery following neoadjuvant therapy for locally advanced ESCC during January 1st, 2013 and December 31st, 2015 in Fudan University Shanghai Cancer Center (FUSCC), and evaluated the influence of neoadjuvant therapy on postoperative events and the influence on disease-free survival (DFS) and overall survival (OS). The study has been approved by the Ethics Committee of FUSCC.
Methods
Patients
We retrospectively reviewed all of the patients underwent surgery following neoadjuvant therapy for locally advanced ESCC during January 1st, 2013 and December 31st, 2015 in FUSCC. Patients were restaged according to the 6th TNM Stage System (9). Locally advanced ESCC was defined as cT3–4 or cN1. Recent years in our center, patients with M1a including supraclavicular node involvement and/or celiac node involvement usually received neoadjuvant therapy followed by surgery.
With respect to pretherapeutic workup, all patients underwent a complete history, physical examination and biochemistry tests. This consisted of an upper gastrointestinal endoscopy, a chest enhanced computed tomography (CT) and ultrasonography (US). For patients with some particular symptoms or signs, further examination including positron emission tomography computed tomography (PET-CT) was applied. Physical condition was evaluated by pulmonary function test, electrocardiogram, cardiac sonography, and so on. Patients without complete pretherapeutic workup in FUSCC were excluded from the study.
Treatment and evaluation
In China, there was no neoadjuvant therapy based upon high-level evidence for locally advanced esophageal cancer. The chemotherapy regimen was usually based on platinum coordination combined with 5-fluorourail (5-FU). The combination with paclitaxel (PTX) or docetaxel (DOC) was also frequently applied. The radiotherapy dose was usually 41.4–50.4 Gy delivered in each fraction of 1.8 or 2 Gy. Intensity modulated radiotherapy (IMRT) were planned by a CT simulator. If both chemotherapy and radiotherapy were administrated, then they were conducted concurrently. After completion of neoadjuvant therapy, patients received a series of workup as above to evaluate the responses and to determine whether surgery would be performed.
Surgery was regularly scheduled at about 4 weeks after the completion. In this study, patients received two-field lymphadenectomy (2FLND) or three-field lymphadenectomy (3FLND), including Ivor-Lewis and McKeown procedures. Cervical node dissection was conducted by a collar incision. The procedures were as previously reported (10,11). R0 resection was defined as microscopically confirmed no tumor cell residual. R1 and R2 resection were defined as microscopically confirmed tumor cell residual and macroscopically confirmed tumor cell residual, respectively.
In this study, patients were restaged according to the 6th TNM stage system. Pathological complete response (pCR) was confirmed when histological analysis of the specimen revealed the absence of tumor cell in both primary lesion and involved nodes (ypT0N0M0). The postoperative ypTNM stage was compared with the pretherapeutic cTNM stage to determine down-staging. Any death within 90 days after the operation or within any time before the hospital discharge was defined as postoperative death.
Follow-up
Patients were usually followed up at our outpatient clinic every 3 months for the first 2 years after the operation, and every 6 months for 3–5 years. Oncological investigations such as CT and US were done during the time. For patients with particular symptoms and signs, additional examinations would be conducted. A combination of clinical service records, phone calls, and letters was used to determine each patient status as of May 2016. A total of 50 patients were included in the study according to the criteria.
Statistical analysis
Statistical analysis was performed by Statistical Package for the Social Sciences software (IBM SPSS version 22, Chicago, IL, USA). The survival curve was calculated by the Kaplan-Meier method. In this study, the OS was defined as the interval between the date of neoadjuvant therapy and the date of death or the date of the last follow-up. DFS was defined as the interval between the date of neoadjuvant therapy and the date of relapse or date of last follow-up. The log-rank test was used for univariate analyses and Cox proportional hazards model was used for multivariate analyses. Statistical analysis was considered to be significant when the probability value (P value) was less than 0.05 (P<0.05).
Results
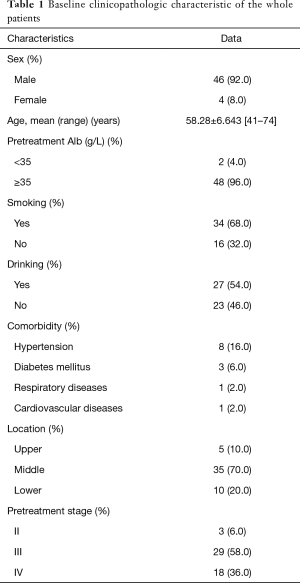
During the studied period, 79 patients received surgery after neoadjuvant therapy. According to the inclusion and exclusion criteria, 29 patients were excluded: 21 patients received neoadjuvant therapy in other hospitals; 4 patients were diagnosed with small cell carcinoma, 2 patients with adenocarcinoma, and 2 patients with adenocarcinoma. Eventually, a total of fifty patients were included into the study. All of the patients were confirmed the status of May 2016. The median follow-up time was 26 months. Baseline clinicopathologic characteristics of these patients were shown in Table 1. There were 46 (92.0%) male patients and 4 (8.0%) female patients. The average age of the whole patients was 58.28±6.643 (range, 41–74) years. Only two (4.0%) patients had pretherapeutic Alb below than 35 g/L. Three (6.0%) patients were diagnosed at the clinical stage II, 29 (58.0%) patients were diagnosed at the clinical stage III and 18 (36.0%) patients were diagnosed at the clinical stage IV-lym.
Full table
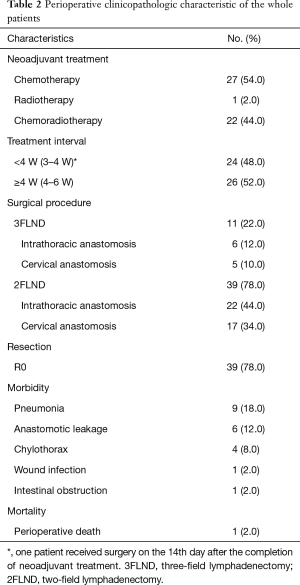
Preoperative clinicopathologic characteristics of the whole patients were shown in Table 2. Twenty-seven (54.0%) patients received neoadjuvant chemotherapy (NCT). Twenty-two (44.0%) patients received concurrent neoadjuvant chemoradiotherapy (NCRT). One (2.0%) patient received neoadjuvant radiotherapy (NRT). The interval between neoadjuvant therapy and surgery was <4 W for 24 (48.0%) patients and ≥4 W for 26 (52.0%) patients. Among patients underwent 2FLND, Ivor-Lewis procedure was performed in 22 (44.0%) patients and McKeown procedure was performed in 17 (34.0%) patients. The remaining 11 (22.0%) patients underwent 3FLND. Thirty-nine (78.0%) patients achieved R0 resection. Morbidities occurred in 16 (32.0%) patients. Pneumonia occurred in 9 (18.0%) patients. Leakage occurred in 6 (12.0%) patients; 5 of the 6 had undergone NCRT, and all of the 6 cases had undergone cervical anastomosis. One (2.0%) patient with anastomotic leakage had postoperative death at about postoperative 90th day.
Full table
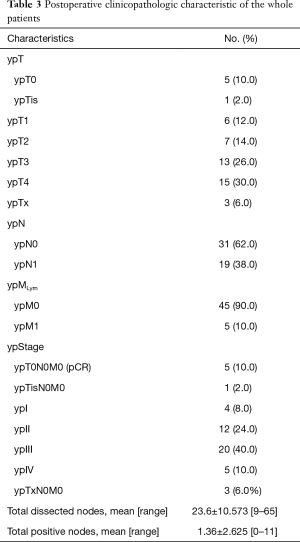
Pathologic characteristics of the whole patients were shown in Table 3. Five (10.0%) patients achieved ypT0N0M0, and 1 (2.0%) patient was classified into the ypTisN0M0 category; all of the 6 patients had undergone NCRT. Three (6.0%) patients could not be precisely classified into a certain ypT category, therefore, they were separately listed. Five (10.0%) patients were classified into ypIV stage due to non-regional lymph node metastasis. The average number of total dissected nodes was 23.6±10.573 (range, 9–65). The average number of total positive nodes was 1.36±2.625 (range, 0–11). The total nodes included both regional and non-regional nodes.
Full table
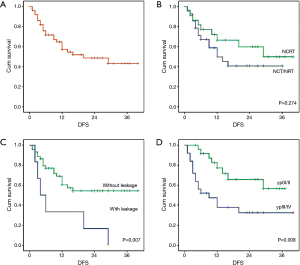
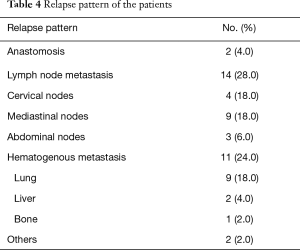
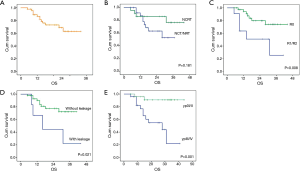
The curve of DFS was shown in Figure 1A. For the whole patients, the 1-, 2- and 3-year DFS rate were 57.0%, 48.0% and 42.0%, respectively. The median DFS period was 20 months. As shown in Table 4, lung metastasis and mediastinal node involvement were the most common relapse patterns. However, in this study tumor relapse was often diagnosed by image examinations instead of pathological biopsy. Univariate analyses and multivariate analyses on DFS were shown in Table 5. Though there was a trend towards better survival in NCRT group, no significant difference was found between NCRT group and NCT/NRT group (Figure 1B, P=0.274). Anastomotic leakage and ypTNM stage shown potential prognostic value for DFS (Figure 1C, P=0.007; Figure 1D, P=0.008, respectively). Eventually, Cox proportional hazards model confirmed both of them as independent prognostic factors for DFS (P=0.021, P=0.017, respectively).
Full table

Full table
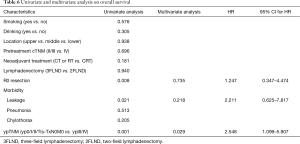
The curve of OS was shown in Figure 2A. For the whole patients, the 1-, 2- and 3-year OS rate were 86.0%, 73.0% and 62.0%, respectively. Univariate analyses and multivariate analyses on DFS were shown in Table 6. Though there was a trend towards better survival in NCRT group, no significant difference was found between NCRT group and NCT/NRT group (Figure 2B, P=0.181). R0 resection, anastomotic leakage and ypTNM stage shown potential prognostic value in log-rank tests (Figure 2C, P=0.008; Figure 2D, P=0.021; Figure 2E, P=0.001, respectively). However, multivariate analysis revealed ypTNM was the independent prognostic factor for OS (P=0.029), and R0 resection and anastomotic leakage was excluded (P=0.218, P=0.735, respectively).
Full table
Discussion
Neoadjuvant therapy followed by surgery has been advocated for patients diagnosed with locally advanced esophageal cancer (1). However, randomized trials did not result in consistent conclusions (2-7). Besides, studies reported that NCRT appeared to achieve better local control but did not significantly affect survival (12,13). It has been recognized that ESCC has different histological and clinical characteristics compared with esophageal adenocarcinoma carcinoma (EAC) (9,14,15). In China, ESCC predominated in all cases (8).
In our study, all patients were pretherapeutically confirmed as locally advanced ESCC according to the 6th edition TNM stage system (9). The 1-, 2-, 3-year DFS and OS rates were 57.0%, 48.0%, 42% and 86.0%, 73.0%, 62.0%, respectively. The results were comparable with previous studies. In 2015, Hamai et al. reported that for the 38 patients received NCRT followed by surgery, 1-, 3-, 5-year DFS and OS were 63.2%, 44.7%, 39.5% and 84.2%, 50.0%, 44.7%, respectively (16). The CROSS study reported that 1-, 2-, 3-year survival rate were 82%, 67%, 58% in the neoadjuvant arm and 70%, 50%, 44% in the surgery alone arm (7). Given to the inclusion of patients with M1, we reviewed literature and found that Miyata and his colleagues reported that for patients with supraclavicular node metastasis before NCT but not after NCT, 3-year survival was 64.9% (17). But the study did not clearly report 3-year survival rate for the whole patients. Neoadjuvant therapy seemed to suggest survival benefits for patients with locally advanced ESCC.
Twelve cases (24.0%) had hematogenous metastasis in our study, which was comparable to that of the CROSS study (28.0%) (18). We found lung metastasis (18.0%) and mediastinal node involvement (18.0%) were the most common relapse patterns. However, in this study tumor relapse was often diagnosed by image examinations including CT, US and tumor markers. 18F-fluorodeoxy glucose positron emission tomography was used if necessary. We considered that the actual events could be lower due to false positive.
The incidence of morbidities was comparable with Hamai and colleagues’ study as above (16). What was notable was that all of the six patients with leakage had undergone NCRT and cervical anastomosis. Some studies suggested that NCRT was a risk factor for morbidities and mortalities (19,20), while some others hold the opposed idea (21-23). A retrospective study from our center reported that cervical leakage occurred more frequently than intrathoracic leakage (23.6%, 145/615 vs. 5.0%, 101/2,023, P<0.001) (24). We also noticed that among the six case, three patients had pneumonia.
Univariate analyses suggested that anastomotic leakage was associated with DFS and OS (P=0.007, P=0.021, respectively). Multivariate analyses revealed that leakage was an independent prognostic factor for DFS (P=0.021) and it was not an independent prognostic factor for OS (P=0.218). However, Kataoka and colleagues reported that leakage was not a prognostic factor (25). They found postoperative infectious complications like pneumonia worsened prognosis. Some other researchers found that postoperative morbidities did not affect long-term survival (23). As above, we noticed that among the six cases, three patients had pneumonia. And given to the ypTNM stage, four of the six patients should have adjuvant therapy due to T3-4 or N+ according to the regular practice in our center (26). In our study, univariate and multivariate analyses confirmed that only ypTNM stage was the independent factor for both DFS and OS. Patients with ypIII or ypIV had a significantly poorer survival. Some studies have demonstrated the prognostic value of postoperative pathological findings including ypTNM (27,28), while other proposed that both pretherapeutic factors also worked as prognostic factors (29,30). Among these prognostic factors, postoperative pathological findings attracted more attention.
Our study has some limitations. During the studies period, more than 1,500 patients received surgery in our center, but very few patients received neoadjuvant treatment. The study includes only 50 patients according to the criteria. Due to the lack of well-recognized principles in China, these patients did not receive totally consistent neoadjuvant therapy. The present retrospective study provided an opportunity to understand safety and survival benefit of patients underwent neoadjuvant therapy followed by surgery. We think it is of some importance because of the lack of data from China.
In conclusion, neoadjuvant therapy did not increase postoperative morbidities but did achieve favorable survival. The ypTNM stage was an independent prognostic factor for both DFS and OS. Long-term survival need further investigation.
Acknowledgements
The authors gratefully acknowledge the valuable cooperation of Ben Ma (Department of Head and Neck Surgery, Fudan University Shanghai Cancer Center, Shanghai, China) and Yangle Huang (Department of Thoracic Surgery, Fudan University Shanghai Cancer Center, Shanghai, China).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The retrospective study has been approved by the Ethics Committee of Fudan University Shanghai Cancer Center (110497-3).
References
- Esophageal and esophagogastric junction cancers, nccn guidelines, version 1.2016. Available online: http://www.nccn.org/
- Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979-84. [Crossref] [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [Crossref] [PubMed]
- Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol 2007;25:3719-25. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062-7. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Sobin LH, Wittekind C, editors. TNM Classification of Malignant Tumours, 6th edition. New York: John Wiley & Sons; 2002.
- Li H, Zhang Y, Cai H, et al. Pattern of lymph node metastases in patients with squamous cell carcinoma of the thoracic esophagus who underwent three-field lymphadenectomy. Eur Surg Res 2007;39:1-6. [Crossref] [PubMed]
- Li H, Yang S, Zhang Y, et al. Thoracic recurrent laryngeal lymph node metastases predict cervical node metastases and benefit from three-field dissection in selected patients with thoracic esophageal squamous cell carcinoma. J Surg Oncol 2012;105:548-52. [Crossref] [PubMed]
- Klevebro F, Alexandersson von Döbeln G, Wang N, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol 2016;27:660-7. [Crossref] [PubMed]
- Samson P, Robinson C, Bradley J, et al. Neoadjuvant Chemotherapy versus Chemoradiation Prior to Esophagectomy: Impact on Rate of Complete Pathologic Response and Survival in Esophageal Cancer Patients. J Thorac Oncol 2016;11:2227-37. [Crossref] [PubMed]
- Sobin LH, Gospodarowicz M, Wittekind C. editors. UICC International union against cancer. Tnm classification of malignant tumors. 7th ed. New York, NY: Wiley-Blackwell; 2009.
- Rice TW, Blackstone EH. Esophageal cancer staging: past, present, and future. Thorac Surg Clin 2013;23:461-9. [Crossref] [PubMed]
- Hamai Y, Hihara J, Emi M, et al. Results of Neoadjuvant Chemoradiotherapy With Docetaxel and 5-Fluorouracil Followed by Esophagectomy to Treat Locally Advanced Esophageal Cancer. Ann Thorac Surg 2015;99:1887-93. [Crossref] [PubMed]
- Miyata H, Yamasaki M, Miyazaki Y, et al. Clinical Importance of Supraclavicular Lymph Node Metastasis After Neoadjuvant Chemotherapy for Esophageal Squamous Cell Carcinoma. Ann Surg 2015;262:280-5. [Crossref] [PubMed]
- Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385-91. [Crossref] [PubMed]
- Kumagai K, Rouvelas I, Tsai JA, et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg 2014;101:321-38. [Crossref] [PubMed]
- Takahata R, Ono S, Tsujimoto H, et al. Preoperative chemoradiation therapy for esophageal cancer is a risk factor for the elevation of high mobility group box-1, leading to an increase in postoperative severe pulmonary complications. Dis Esophagus 2016;29:70-8. [Crossref] [PubMed]
- Gronnier C, Tréchot B, Duhamel A, et al. Impact of neoadjuvant chemoradiotherapy on postoperative outcomes after esophageal cancer resection: results of a European multicenter study. Ann Surg 2014;260:764-70; discussion 770-1. [Crossref] [PubMed]
- Hamai Y, Hihara J, Taomoto J, et al. Effects of neoadjuvant chemoradiotherapy on postoperative morbidity and mortality associated with esophageal cancer. Dis Esophagus 2015;28:358-64. [Crossref] [PubMed]
- Lindner K, Fritz M, Haane C, et al. Postoperative complications do not affect long-term outcome in esophageal cancer patients. World J Surg 2014;38:2652-61. [Crossref] [PubMed]
- Li B, Xiang J, Zhang Y, et al. Factors Affecting Hospital Mortality in Patients with Esophagogastric Anastomotic Leak: A Retrospective Study. World J Surg 2016;40:1152-7. [Crossref] [PubMed]
- Kataoka K, Takeuchi H, Mizusawa J, et al. Prognostic Impact of Postoperative Morbidity After Esophagectomy for Esophageal Cancer: Exploratory Analysis of JCOG9907. Ann Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Zhu Z, Yu W, Li H, et al. Nodal skip metastasis is not a predictor of survival in thoracic esophageal squamous cell carcinoma. Ann Surg Oncol 2013;20:3052-8. [Crossref] [PubMed]
- Kim YH, Song SY, Shim HJ, et al. Treatment outcomes of neoadjuvant concurrent chemoradiotherapy followed by esophagectomy for patients with esophageal cancer. Radiat Oncol J 2015;33:12-20. [Crossref] [PubMed]
- Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol 2014;32:2983-90. [Crossref] [PubMed]
- Yokota T, Ando N, Igaki H, et al. Prognostic Factors in Patients Receiving Neoadjuvant 5-Fluorouracil plus Cisplatin for Advanced Esophageal Cancer (JCOG9907). Oncology 2015;89:143-51. [Crossref] [PubMed]
- Kobayashi T, Teruya M, Kishiki T, et al. Inflammation-based prognostic score, prior to neoadjuvant chemoradiotherapy, predicts postoperative outcome in patients with esophageal squamous cell carcinoma. Surgery 2008;144:729-35. [Crossref] [PubMed]


