Chronic pulmonary mucormycosis: an emerging fungal infection in diabetes mellitus
Case 1
A 60-year-old diabetic female presented with fever and productive cough with hemoptysis for two months. There was no significant past medical or surgical history. Crackles were audible at right middle and lower part of chest. Rest of the physical examination was unremarkable.
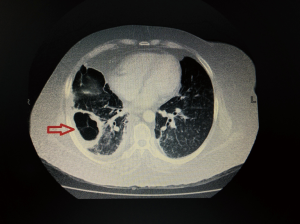
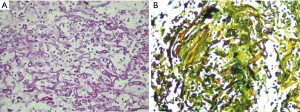
On laboratory investigations, total leucocyte count (TLC) was 13.1×109/L with 73.6% neutrophil, potassium (K) was 5.5 mg/dL, creatinine was 1.1 mg/dL. Glycated hemoglobin (HbA1c) was 13.1%. Serial chest X-rays showed persistent homogenous consolidation in right lower lung (Figure 1A). So computed tomographic (CT) scan chest was done which showed a consolidation with cavitary lesion in right middle lobe (Figure 1B). Fibre-optic bronchoscopy revealed anthracosis of right middle lobe, biopsy and bronchoalveolar lavage (BAL) was taken. BAL was negative for acid fast bacilli (AFB), fungal smear showed numerous aseptate hyphae which later failed to grow. Histopathology revealed large collections of aseptate hyphae infiltrating the lung parenchyma, periodic acid-Schiff and Gomori methenamine silver stains were positive suggestive of pulmonary Mucor (Figure 2A,B). She was started on amphotericin B deoxycholate 1.5 mg/kg/dose and underwent right middle lobectomy. Patient remained well and discharged home on amphotericin. She received a total cumulative dose of 3 grams of amphotericin B deoxycholate. Her medications were adjusted to achieve good glycaemic control and showed significant improvement on clinic follow ups.
Case 2
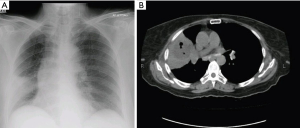
A 60-year-old diabetic female presented with history of cough and fever for one month. She had taken multiple antibiotics without any improvement. Crackles were audible in left middle to lower part of chest. Complete blood count (CBC) showed TLC of 11.8×109/L with 68.8% neutrophils. Random blood sugar was 284 mg/dL and HbA1c was 11.4%. Chest X-ray showed left hilar soft tissue mass with perihilar infiltrates (Figure 3A). High-resolution CT chest (HRCT) showed consolidation in lingular segment and left upper lobe of lung with few surrounding scattered infiltrates (Figure 3B). She underwent flexible bronchoscopy which showed narrowing of left upper lobe and anthracosis. BAL was negative for AFB smear and culture and fungal smear and culture. Histopathology revealed aseptate hyphae suggestive of mucormycosis. She was started on amphotericin B deoxycholate 1.5 mg/kg/dose. Left pneumonectomy was done due to intense infiltration of lung hilum involving left main bronchus and pulmonary vessels. Histopathology showed lung parenchyma exhibiting numerous aseptate hyphae. She was discharged home and amphotericin B deoxycholate was continued till cumulative dose of 3 grams. She responded well to treatment and remained stable on clinic follow ups.
Case 3
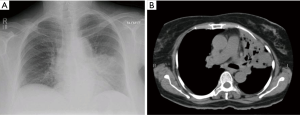
A 69-year-old diabetic female presented with shortness of breath, productive cough and fever for two month. Bilateral crackles were audible on lower chest. CBC showed TLC 8.4×109/L with 76.6% neutrophils. Serum K was 2.4 mmol/L, creatinine was 0.5 mg/dL and blood urea nitrogen was 14 mg/dL. Her HbA1c was 9.4%. Chest X-ray showed bilateral lower zone alveolar infiltrates and cystic changes in right lower zone in perihilar region. CT scan chest with contrast showed filling defect at bifurcation of right pulmonary artery consistent with pulmonary embolism. This was associated with patchy airspace shadowing, apical fibrosis, fibrocystic and fibrocavitary changes in lungs more marked on right side (Figure 4). Bronchoscopy was done and fungal culture yielded Rhizopus species. She was started on amphotericin B deoxycholate 1.5 mg/kg/dose. Surgical opinion was sought but was not advisable due to bilateral lung involvement. She responded to medical treatment and was discharged home on amphotericin B deoxycholate and therapeutic anticoagulation therapy but later lost to follow up.
Discussion
Pulmonary mucormycosis (PM) is a life threatening condition, if left undiagnosed and untreated. Mostly its presentation is acute while chronic presentation is rarely seen suggested that it may have an indolent course particularly in diabetes (1-3). Therefore chronicity of symptoms does not rule out PM. In a retrospective review of mucormycosis by Lee et al. [1999] (1) found that DM was the most common risk factor, of 86 cases 56% patients were diabetic. Various PM cases have been reported from India, where PM has presented as mimicking tuberculosis (TB) (3), as non-resolving pneumonia (2,4) and as co-infection with TB (5). The incidence of PM in India was found 2.5% in a prospective study of 38 patients by Bala et al. [2015] (6). Limited data from Pakistan is available on rhino-cerebral mucormycosis but no PM cases have been reported yet.
PM occurs most commonly in neutropenic patients with leukemic disorders or undergoing HSCT. In epidemiological review of 929 patients with zygomycosis by Roden et al., the incidence of Mucor sinus infection was more common followed by pulmonary and cutaneous. The overall mortality of mucormycosis in diabetes was 44% whereas with PM mortality increased to 76% (7).
There are no specific sign and symptoms of PM (1) and difficult to distinguish from other fungal infection like aspergillus. Radiographically, it may present as lobar consolidation, mass, nodular infiltrates, cavitation, infarcts due to thrombosis of the pulmonary vessels by fungal angioinvasion (1,8). The presence of air crescent sign may be associated with massive hemoptysis. The “reversed halo sign” (“central ground glass opacity with surrounded by dense air-space consolidation”) on the CT scan is observed in immune compromised hosts with PM (9) but it is also nonspecific and can be seen in pulmonary aspergillosis.
Endo-bronchially it can cause stenosis, airways obstruction, erythematous mucosa to fungating polypoid mass (1). While diabetic patients have more predilection for endobronchial disease (10), our patients presented with non-specific symptoms, in the absence of any other risk factor except diabetes so our first differential diagnosis was TB. Endobronchially, these patients had anthracosis pigmentation in involved lung segment while one had underlying stenosis too.
Unlike invasive aspergillosis, there are no validated biomarkers for the diagnosis of mucormycosis. Due to non-specific clinical presentation, a high index of suspicious is always required, a clinician must keep this entity in mind especially in diabetic patients and pursue invasive testing early to establish a prompt diagnosis. The European Society for Clinical Microbiology and Infectious Diseases and European Confederation of Medical Mycology ESCMID and ECMM Joint, clinical guidelines strongly recommend direct microscopy, histopathology and culture for the diagnosis (11).
The diagnosis of PM is more difficult than other fungal infections and histopathology is more sensitive than cultures (1). Mucorales are rapidly growing fungi, but the yield of cultures is low and could be due to aggressive processing of the specimen or prior antifungal therapy. Direct microscopy of the specimens is an important diagnostic tool, since it distinguishes between a pathogen and contaminant (12). Identification on H&E staining is also difficult and many times special staining techniques are required for diagnosis. The demonstration of fungi on cytological examination may be difficult due to the difficulty in extracting fungal elements from tissues, fungal elements may be rare in cytologic specimens and when present are often fragmented, very focal and may appear in only part of the specimen (13). It is important to note that the key feature of Mucorales is that it produce wide ribbon-like aseptate hyphae in tissues with branching occurs at wide angles nearing 90° while Aspergillus spp. produce thin septate hyphae branch at acute angles of 45°. Two out of three cases in our series failed to grow on culture despite positive microscopy may be because of above mentioned reason and diagnosis was based on histopathology. This highlights the low sensitivity of culture and points towards significance of alternate diagnostic methods. Recently in a study of 204 patients, it was found that Mucorales-specific T cells can be detected and monitored in patients with hematologic malignancies and may be evaluated as a surrogate diagnostic marker (14), however no studies have performed in diabetic or other population.
Management of PM requires surgical intervention, and correction of underlying predisposing factors. Medical therapy includes lipid formulation of amphotericin B and posaconazole as step-down or as salvage therapy (11,15). Surgical intervention improves the overall prognosis (16), even in hematopoietic disorder patients (17). Surgical debridement, in addition to immediate first-line antifungal treatment has significant better outcomes compared to antifungal alone and it is strongly recommended (1,6). Two of our patients underwent surgical intervention. One patient with local PM underwent lobectomy and second patient had pneumonectomy because of risk of dissemination. Third patient did not undergo surgery because of bilateral parenchymal abnormalities. Patients responded well to treatment and are in follow up except for one patient who was lost to follow up. These are first cases reported from Pakistan on chronic PM in diabetes and suggest that the chronic PM is uncommon but should be considered in patients with DM. Prompt diagnosis can improve outcome in these patients.
Conclusions
PM is an emerging cause of infectious morbidity and mortality. However, there is no specific sign and symptoms, diagnostic criteria and specific antigen test for the diagnosis and management of PM. As the incidence of DM is rising both in developed and developing countries it is important need to raise awareness regarding PM. Any history of fever non-responding to antibiotics with pulmonary signs should also raise the suspicion of fungal infection like PM.
Acknowledgements
The authors thank Dr. Saulat Fatimi, cardiothoracic surgeon who did surgery in both patients.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Lee FY, Mossad SB, Adal KA. Pulmonary mucormycosis: the last 30 years. Arch Intern Med 1999;159:1301-9. [Crossref] [PubMed]
- Agarwal R, Kumar V, Gupta D. Pulmonary mucormycosis: two of a kind. Eur J Intern Med 2006;17:63-5. [Crossref] [PubMed]
- Garg R, Marak RS, Verma SK, et al. Pulmonary mucormycosis mimicking as pulmonary tuberculosis: a case report. Lung India 2008;25:129-31. [Crossref] [PubMed]
- Panigrahi MK, Manju R, Kumar SV, et al. Pulmonary mucormycosis presenting as nonresolving pneumonia in a patient with diabetes mellitus. Respir Care 2014;59:e201-5. [Crossref] [PubMed]
- Aggarwal D, Chander J, Janmeja AK, et al. Pulmonary tuberculosis and mucormycosis co-infection in a diabetic patient. Lung India 2015;32:53-5. [Crossref] [PubMed]
- Bala K, Chander J, Handa U, et al. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Med Mycol 2015;53:248-57. [Crossref] [PubMed]
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634-53. [Crossref] [PubMed]
- McAdams HP, Rosado de Christenson M, Strollo DC, et al. Pulmonary mucormycosis: radiologic findings in 32 cases. AJR Am J Roentgenol 1997;168:1541-8. [Crossref] [PubMed]
- Wahba H, Truong MT, Lei X, et al. Reversed halo sign in invasive pulmonary fungal infections. Clin Infect Dis 2008;46:1733-7. [Crossref] [PubMed]
- Husari AW, Jensen WA, Kirsch CM, et al. Pulmonary mucormycosis presenting as an endobronchial lesion. Chest 1994;106:1889-91. [Crossref] [PubMed]
- Cornely OA, Arikan-Akdagli S, Dannaoui E, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect 2014;20 Suppl 3:5-26. [Crossref] [PubMed]
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev 2011;24:247-80. [Crossref] [PubMed]
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev 2000;13:236-301. [Crossref] [PubMed]
- Potenza L, Vallerini D, Barozzi P, et al. Mucorales-Specific T Cells in Patients with Hematologic Malignancies. PLoS One 2016;11:e0149108. [Crossref] [PubMed]
- Spellberg B, Walsh TJ, Kontoyiannis DP, et al. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis 2009;48:1743-51. [Crossref] [PubMed]
- Tedder M, Spratt JA, Anstadt MP, et al. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg 1994;57:1044-50. [Crossref] [PubMed]
- Moon Y, Park JK, Sung SW. Surgery for localized pulmonary mycotic infections in patients with hematopoietic disorder. J Cardiothorac Surg 2015;10:91. [Crossref] [PubMed]