Bronchoscopic valves for prolonged air leak: current status and technique
Introduction
Unidirectional airway valves are devices used for the treatment of persistent air leaks (PALs) secondary to alveolar-pleural fistulas (APF) or bronchopleural fistulas (BPFs). These airway valves are placed in proximal airways leading to fistulous lung segments via conventional flexible bronchoscopy (Figure 1). Following placement, airflow through fistulous lung segments is reduced, allowing the defect to heal.
Conservative treatments for PALs revolve primarily around prolonged chest tube drainage. Patients suffering from PALs have increased risk of intensive care unit (ICU) readmission, pneumonia, and extended hospital stays (1,2). Invasive approaches to PALs secondary to BPFs and APFs include mechanical and/or chemical pleurodesis, fibrin sealants, bronchial stump re-stapling, muscle flap coverage, and lung resection. Airway valves are being increasingly utilized given their effectiveness in resolving air leaks, thus shortening duration of hospitalizations, reducing the need for operative intervention, and minimizing the risk of hospital associated complications (3).
Development of airway valves
Unidirectional airway valves were originally developed as a non-surgical alternative to lung volume reduction surgery (LVRS) for patients with chronic obstructive pulmonary disease (COPD). The use of airway valves for the treatment of emphysema aimed to emulate the survival benefits demonstrated in the National Emphysema Treatment Trial (NETT) for surgical LVRS in patients with upper lobe predominant disease and poor exercise capacity (4). Two different valve designs were tested in studies for bronchoscopic LVRS: the Zephyr EBV (Pulmonx) and the Spiration intrabronchial valve (IBV) (Olympus Corporation of the Americas). In separate studies, the Zephyr EBV and Spiration IBV were used to occlude the bilateral upper lobe segmental airways to induce atelectasis of diseased parenchyma. This resulted in improved diaphragmatic motion and reduced ventilation-perfusion mismatching.
Initial bronchoscopic LVRS trials for the Zephyr EBV and Spiration IBVs did not provide adequate evidence for the Federal Drug Administration (FDA) approval. The endobronchial valve for emphysema palliation trial (VENT) compared the safety and efficacy of Zephyr EBV therapy in patients with heterogeneous emphysema versus standard medical care (5). This trial showed improvement in lung function, exercise tolerance, and dyspnea in the treatment group. Unfortunately, the study did not meet minimum clinically important difference (MCID) defining significance in dyspnea scores. Additionally, patients experienced an increased frequency of COPD exacerbations, pneumonia, and hemoptysis after valve implantation. An early pilot study using Spiration IBVs was an open-label trial targeting 91 patients with severe, bilateral heterogeneous disease with upper lobe predominance (6). Results of this pilot study were notable for sustained benefit in health related quality of life (HRQL) evidenced by improvements in the St. George’s Respiratory Questionnaire (SGRQ). However, the benefits were not statistically significant in forced expiratory volume in 1 second (FEV1), lung volumes by plethysmography, or 6 minute walk distance (6MWD).
Extrapolating from bronchoscopic LVRS trials, in 2008 the FDA approved the Spiration IBV system under a Humanitarian Device Exemption (HDE) for the treatment of PALs due to APF and BPFs. Approval under the HDE is based on the results of a 58-patient U.S. investigational device exemption (IDE) study for the treatment of severe emphysema. During this study, four patients were treated with Spiration IBVs for prolonged air leaks under the IDE compassionate use exemption (1). The patients had multiple medical problems justifying the use of IBVs in post-operative persistent air leaks despite conventional medical therapy. All four patients experienced immediate improvement or resolution of their air leaks. Based on these clinical results, along with ex vivo calf and human lung testing, unidirectional airway valves have become an integral part of the modern treatment algorithm for treatment of PALs.
Spiration IBVs and indications for use
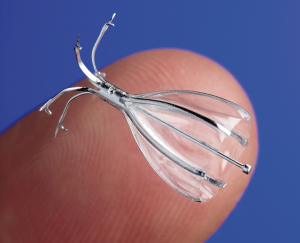
Spiration IBVs are umbrella shaped valves composed of a nitinol frame consisting of anchors, struts, and a polyurethane membrane (Figure 2). The construction of the valve allows it to expand and contract with airway movement during respiration. This intricate design allows the valve to limit airflow distally, while still permitting drainage of secretions into proximal airways. Valves are available in various diameters and lengths depending on the size of the segmental airway to be occluded. While valves can remain in the airways for an unlimited amount of time, they can be removed bronchoscopically.
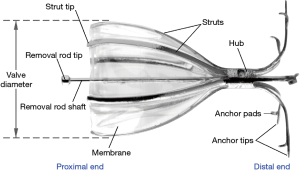
Indications for use of Spiration IBVs include PALs following lobectomy, segmentectomy, or LVRS. An air leak present on postoperative day 7 unless present only during forced exhalation or cough is an indication for the placement IBVs. Additionally, an air leak present on day 5 can be considered appropriate for treatment if it is continuous, present during normal inhalation phase of inspiration, or present upon normal exhalation and accompanied by subcutaneous emphysema or respiratory compromise. While these indications for IBV use are defined by the FDA’s HDE, a large number of Spiration IBVs are placed for off-label uses such as APFs and BPFs following pneumothoraces, pulmonary infections, trauma, and malignancies.
Despite clear indications for the placement of Spiration IBVs for PALs present for 5–7 days following surgery, a large percentage of valves are placed after several week of having a PAL. While cost of the valve placement may be a factor in this delay, observation of daily progressive improvement in the PALs, even minimal, without intervention is the main reason placement of valves is delayed. Physician belief that the PAL will resolve on its own despite many weeks of observation can delay valve placement and result in prolonged hospitalization with increased health care costs. Greater familiarity of the devices along with the awareness of indications would benefits patients suffering from PALs.
Spiration IBV placement technique
The placement of Spiration IBVs involves a multi-step process aimed at identifying and occluding segmental airways harboring APFs or BPFs. These steps involve the use of an airway occluding balloon, sizing balloon, and a specialized valve deployment catheter. A calculated approach to placement allows for effective identification of damaged lung segments and successful deployment of valves.
Patient selection and preparation are essential to ensure accurate air leak localization and IBV placement. The patient must have a functioning chest tube in the affected pleural space with the application of wall suction. Use of general anesthesia and endotracheal intubation are recommended due to the length of the procedure and to ensure a closed circuit of air flow within the chest. Patients must be able to tolerate positive pressure ventilation and have adequate pulmonary reserve to tolerate the loss of lung volume due to induced atelectasis after valve placement.
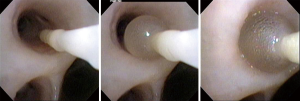
Methodical airway occlusion is the initial step for IBV placement. Localization of the proximal airway leading to the affected segment is the most time-consuming portion of valve placement. Segmental airways potentially leading to fistulous lung segments are occluded using the sizing balloon (or fogarty balloon) from the most proximal to most distal airways (Figure 3). Following airway occlusion, visualization of the leak chamber contained in the chest tube collection system is necessary to detect any reduction of bubbling. Decreased bubbling in the leak chamber represents reduction in air flow through the fistula and correct identification of the effected segment. Four to five respiratory cycles should be observed following airway occlusion to washout any residual air in the lung or pleural space. A reduction in the size of the leak may indicate that the operator is following the correct path to the involved segment, while an increase in the air leak may represent redistribution of more air from a normal segment into the segment containing the defect.
Once the offending segmental airway is identified, the pre calibrated sizing balloon is used to determine the appropriate valve diameter for occlusion. Once the balloon has been inflated and makes circumferential contact with all portions of the airway wall, the balloon should be advanced and withdrawn gently to ensure correct sizing. The volume needed to achieve circumferential contact should be used to identify the size of the valve to be used.
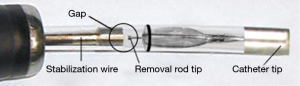
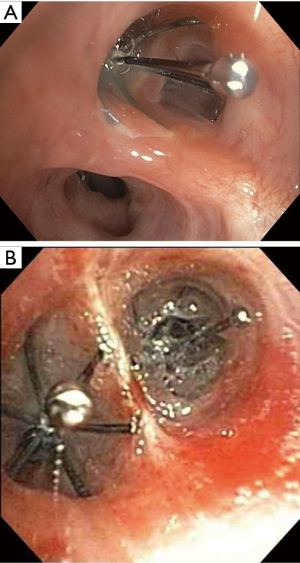
The actual placement of Spiration IBVs requires a specialized deployment catheter. Following accurate sizing of the airway, the chosen diameter valve is loaded into the deployment catheter. The deployment catheter is then fed through the working channel of the bronchoscope until visible within the airways. Prior to placement of the valve, the operator must “close the gap” between the removal rod and stabilization wire to improve deployment accuracy and prevent kinking of the catheter (Figure 4). The deployment catheter is then advanced into the desired airway and is positioned at the most proximal lip of the segment (Figure 5). During maximal inhalation, the retractor handle should be squeezed with persistent pressure to deploy the IBV over a period of 1 to 2 seconds. After successful deployment and airway occlusion, other segments should be tested in the same manner to assess need for additional valve placement. The goal of the procedure is to attain significant reduction in the degree of the air leak or resolution. Resolution of the air leak is typically observed after an average of 4.5 days (3).
Following successful resolution of the PAL via Spiration IBV placement adequate time for tissue healing is necessary. This time period is approximately 6 weeks in most patients. Valve removal should be performed bronchoscopically with the aid of flexible bronchoscopy forceps. The removal rod shaft located on the valve can be grasped using flexible forceps and withdrawn through the endotracheal tube. Once all valves are removed a chest X-ray should be performed to confirm that the pneumothorax does not recur.
Clinical literature supporting the use of Spiration IBVs for pals
The literature supporting the use of unidirectional airway valves for the bronchoscopic treatment of PALs is grounded primarily in case reports. Initial case reports describing use of unidirectional airway valves in animals and humans utilized the Zephyr EBV. The first animal studies involved surgically induced APFs with resulting PALs in six sheep (7). Zephyr EBVs were bronchoscopically deployed into the intentionally injured segmental airway with immediate resolution of the air leak. Repeat bronchoscopy at 1 week and 4 weeks showed no change in the device location. A number of trials have featured the use of Zephyr EBV valves in humans (8-11). Notably, Travaline et al. featured the use of Zephyr EBVs for PALs in a multicenter trial which enrolled 40 patients from 17 international sites (12). The etiology of the PALs were recurrent spontaneous pneumothorax in 21 patients, postoperative complications in 7 patients, iatrogenic in 6 patients, first time pneumothorax in 4 patients, and from bronchoscopic LVRS in 1 patient. Following bronchoscopic EBV placement, 19 patients had complete resolution of their PALs, 18 patients had a reduction in their PALs, 2 had no change, and 1 had no reported outcome. Resolution of the air-leak was measured by time to chest tube removal following EBV placement. Mean time to chest tube removal was 21 days [median, 7.5 days; interquartile range (IQR), 3 to 29 days] with a mean time to discharge from the hospital of 19 days (median, 11 days; IQR, 4 to 27 days).
While early trials testing the use of unidirectional valve treatments for PALs featured the Zephyr EBVs, FDA approval was only granted for the use of Spiration IBVs under the HDE based on data from the U.S. IDE study. The most notable case series investigating the benefit of Spiration IBVs in PALs involved seven patients performed by Gillespie et al. (3). Seven patients suffering from complex alveolar fistulas were treated over a 15-month period with 8 valve placement procedures. All patients displayed an improvement in PALs. The median air leak duration prior to intervention was 4 weeks. After Spiration IBV placement, the median air leak duration was only 1 day with a mean duration of 4.5 days. Discharge within 2 to 3 days of the procedure occurred in 57% of patients. The median number of valves placed was 3.5 and all valves were removed successfully after 6 weeks. No procedural or valve related complications were observed.
A handful of additional case series highlight the safety and effectiveness of Spiration IBVs (13,14). The use of Spiration IBVs was described by Mahajan et al. for the treatment of three patients with respiratory failure secondary to PALs (15). This case series described a reduction in the need for significant ventilator support and high oxygen requirement in ICU patients with severe PALs. Additionally, Spiration IBVs have been used in APFs following pulmonary infections such as pneumocystis jiroveci (16). Based on the design of the Spiration IBV, which allow for proximal drainage of secretions, these valves could theoretically be used in suppurative pulmonary infections complicated with a PAL as well. We favor delaying IBV placement until clinical improvement is observed with tube thoracostomy and antibiotic therapy.
While case series are the main source of literature supporting the use of Spiration IBVs, no randomized studies have been published showing effectiveness. The valves against standard therapy (VAST) is a multicenter, prospective, randomized trial aimed at evaluating the use of Spiration IBVs in the treatment of PALS versus standard therapy. This study hopes to provide much needed data to help define the role of Spiration valves in APFs and BPFs.
Conclusions
Unidirectional airway valves are becoming successfully integrated into the treatment algorithm for the treatment of PALs. The indication for Spiration IBV placement has been clearly defined under the HDE but are regularly placed off-label. Placement techniques remain operator and patient friendly and allow the procedure to be performed with relative ease. While the current body of literature available to justify the use of unidirectional valves is limited to case series, current multicenter, randomized trials should provide further guidance regarding patient selection and treatment efficacy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Liberman M, Muzikansky A, Wright CD, et al. Incidence and risk factors of persistent air leak after major pulmonary resection and use of chemical pleurodesis. Ann Thorac Surg 2010;89:891-7. [Crossref] [PubMed]
- DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg 2006;82:197-206. [Crossref] [PubMed]
- Gillespie CT, Sterman DH, Cerfolio RJ, et al. Endobronchial valve treatment for prolonged air leaks of the lung: a case series. Ann Thorac Surg 2011;91:270-3. [Crossref] [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. [Crossref] [PubMed]
- Sterman DH, Mehta AC, Wood DE, et al. A multicenter pilot study of a bronchial valve for the treatment of severe emphysema. Respiration 2010;79:222-33. [Crossref] [PubMed]
- Fann JI, Berry GJ, Burdon TA. The use of endobronchial valve device to eliminate air leak. Respir Med 2006;100:1402-6. [Crossref] [PubMed]
- Mitchell KM, Boley TM, Hazelrigg SR., et al. Endobronchial valves for treatment of bronchopleural fistula. Ann Thorac Surg 2006;81:1129-31. [Crossref] [PubMed]
- Ferguson JS, Sprenger K, Van Natta T. Closure of a bronchopleural fistula using bronchoscopic placement of an endobronchial valve designed for the treatment of emphysema. Chest 2006;129:479-81. [Crossref] [PubMed]
- Anile M, Venuta F, De Giacomo T, et al. Treatment of persistent air leakage with endobronchial one-way valves. J Thorac Cardiovasc Surg 2006;132:711-2. [Crossref] [PubMed]
- Feller-Kopman D, Bechara R, Garland R, et al. Use of a removable endobronchial valve for the treatment of bronchopleural fistula. Chest 2006;130:273-5. [Crossref] [PubMed]
- Travaline JM, McKenna RJ Jr, De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest 2009;136:355-60. [Crossref] [PubMed]
- El-Sameed Y, Waness A, Al Shamsi I, et al. Endobronchial valves in the management of broncho-pleural and alveolo-pleural fistulae. Lung 2012;190:347-51. [Crossref] [PubMed]
- Reed MF, Gilbert CR, Taylor MD, et al. Endobronchial Valves for Challenging Air Leaks. Ann Thorac Surg 2015;100:1181-6. [Crossref] [PubMed]
- Mahajan AK, Verhoef P, Patel SB, et al. Intrabronchial valves: a case series describing a minimally invasive approach to bronchopleural fistulas in medical intensive care unit patients. J Bronchology Interv Pulmonol 2012;19:137-41. [Crossref] [PubMed]
- Vicencio AG, Tozzi M, Thompson C, et al. Intrabronchial valves for treatment of alveolar-pleural fistula in a patient with Pneumocystis jirovecii pneumonia. J Bronchology Interv Pulmonol 2014;21:346-9. [Crossref] [PubMed]