Immunity status of invasive pulmonary aspergillosis patients with structural lung diseases in Chinese adults
Introduction
Aspergilli are respiratory pathogens acquired through the inhalation of Aspergillus conidia, the spores of which are universally present in unfiltered air. While generally not harmful for immunocompetent hosts, colonization and infection of the lungs can occur in those with weakened immune systems, leading to the development of invasive pulmonary aspergillosis (IPA). As the disease is difficult to diagnose early and lacks effective treatment options, the survival rate of IPA patients is very poor.
IPA is well-recognized as a severe fungal lung infection, frequently observed in patients following procedures such as allogeneic bone marrow transplantation and solid organ transplantation, as well as alongside conditions including hematologic malignancies, late-stage HIV infection, and chronic granulomatosis (1-3). The disease has also grown in prevalence as a comorbidity in patients with structural lung diseases such as chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD), and non-cystic fibrosis bronchiectasis (NCFB)—all of which confer different immune dysfunctions to their patients.
Due to the significance of immunology in fungal infection, IPA has been an area of focus for many experts. Several studies have described an increase of IPA incidence in COPD patients (3-7); however, IPA assessments in relation to other common respiratory diseases remain scarce, particularly those evaluating patient immune status. Therefore, this study aims to assess the immune status of IPA patients with COPD, ILD, and NCFB while excluding the presence of basic diseases.
Methods
Study design and diagnostic procedure
Our study retrospectively assessed patients treated at Shanghai Pulmonary Hospital, Tongji University, between 2004 and 2013. Diagnosis of IPA was classified as proven, probable, or possible in accordance with the revised EORTC and MSG criteria (8,9). Consequently, a diagnosis of “proven IPA” was established by both (I) the presence of hyphae compatible with Aspergillus in specimens collected from a pulmonary lesion sample; and (II) isolation of Aspergillus species in cultures from any lower respiratory tract (LRT) sample, mainly sputum and bronchial secretions, including bronchial brush and bronchial alveolar lavage fluid, etc. combined with evidence of associated tissue damage or two sequential positive serum galactomannan (GM) tests.
COPD diagnosis was established according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (10). In patients with COPD, Bulpa criteria were used for IPA diagnosis (5). NCFB diagnosed routinely and confirmed through high-resolution computed tomography (HRCT) chest imaging and patient history of the syndrome (11,12). ILD diagnosis was established according to the American Thoracic Society Clinical Practice Guideline published in 2012 (13).
Inclusion criteria and data collection
We examined the data on Aspergillus isolation from LRT taken at chest and infectious disease clinics in Shanghai Pulmonary Hospital between January 2004 and December 2013. Patient inclusion criteria required both Aspergillus isolation (colonization alone being insufficient), and a diagnosis of either “proven IPA” or “probable IPA”. As only patients with a positive Aspergillus culture were enrolled, no cases of “possible IPA” were obtained. Medical files, discharge reports and CT images from the hospital data processing system were reviewed for each patient in order to obtain data on their demographic features, smoking history, and comorbidities.
Laboratory methods
Following direct microscopic examination, all LRT specimens were cultured on Sabouraud dextrose agar (SDA) either with or without antibiotics. Peripheral blood samples (2.0 mL) were collected in anticoagulation tube containing EDTA to determine CD3+ T lymphocyte, CD3+/CD4+/CD8− lymphocyte (abb.CD4+), CD3+/CD4−/CD8+ lymphocyte (abb.CD8+), CD4+/CD8+ T lymphocyte counts (Shanghai Huizhong Cellular Biotechnology Co., Ltd.). The whole blood samples were also collected to test IgA, IgM and IgG by Immune scatter turbidity method (Siemens Corporation, Germany). All laboratory examinations were performed according to the manufacturers’ directions.
The study was approved by institutional ethics committee board of the Shanghai Pulmonary Hospital (No. K16-322) and was consistent with the principles outlined in the Declaration of Helsinki.
Statistical analyses
All statistical analyses were carried out with SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are described with mean ± SD or median (IQR) for normal distributed variable and skewed distributed variable respectively. Categorical variables are expressed as percentages. Comparisons of serum immune levels among patients with COPD + IPA, ILD + IPA, NCFB + IPA and IPA were made using one-way analysis of variance (ANOVA). P<0.05 was considered statistically significant.
Results
Patient characteristics
In total, aspergillum was isolated and identified as proven IPA in the LRT samples of 77 patients. Among them, forty-seven (61%) were male and 30 (39%) were female, with a mean age of 56. A history of smoking was present in 49 (64%) cases. Thirty-three patients (43%) included reported corticosteroid use over the previous 3 weeks, and 39 (51%) reported frequent antibiotic use (Table 1).
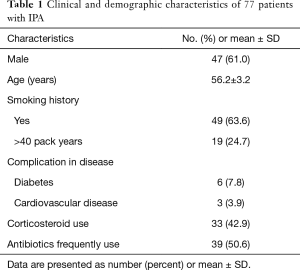
Full table
Of 26 IPA patients with COPD, 23 (89%) used corticosteroids and 18 (69%) used antibiotics. Of 15 IPA patients with ILD, 10 (67%) used corticosteroids and 8 (53%) used antibiotics. Of 15 IPA patients with bronchiectasis, 13 (87%) used antibiotics (Table 2).
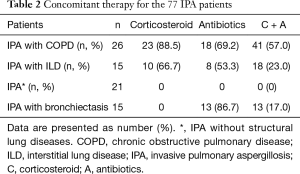
Full table
Cell-mediated immunity analysis
For CD3+ T lymphocyte, there are abundant differences between COPD + IPA, ILD + IPA, NCFB + IPA and IPA (all P<0.001) indicating significant immune differences among these four diseases (Table 3) (Figure 1A).
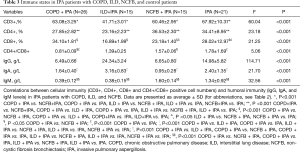
Full table
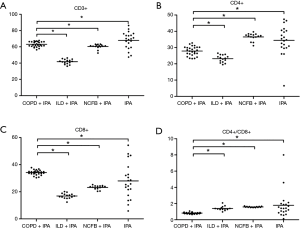
CD4+ T cell and CD8+ T cell are the constituents of CD3+ T cell. From Table 3 and Figure 1B,C, CD4+ and CD8+ T cells also have significant differences between patients with COFP + IPA, ILD + IPA, NCFB + IPA and IPA (P<0.05).
Compared with IPA patients with ILD and NCFB, CD4+/CD8+ double positive cells, a marker of cell-mediated immunity, were less abundant in patients with COPD + IPA [(0.81%±0.09% vs. 1.39%±0.25% and 0.81%±0.09% vs. 1.57%±0.06%), P<0.001, respectively] (Table 3) (Figure 1D). There is trend of increase of cell-mediated immunity status in patients with COPD + IPA, ILD + IPA, NCFB + IPA and IPA (Figure 1D).
Humeral immunity analysis
In with respect to humeral immunity, IgA, IgM and IgG had significant differences between these four diseases (Figure 2A,B,C). IgA levels, which indicate respiratory humoral immunity, were lower in IPA patients with NCFB than those with COPD + IPA and ILD + IPA (0.95±0.28 vs. 1.64±0.4 g/L and 0.95±0.28 vs. 3.16±0.83 g/L, respectively, all P<0.001) (Table 3) (Figure 2B).

Discussion
An increasing number of studies have reported the emergence of IPA in critically ill, immunocompromised patients, whose immune statuses are characterized by multifactorial impairments in their local defenses, i.e., cell-mediated and humoral immunity. The major parameters affecting immunocompetence in these patients are systemic steroid use and the administration of broad-spectrum antibiotics, particularly in those with COPD, ILD, and NCFB.
We found that CD4+/CD8+ positive cells, a marker of cell-mediated immunity, are less abundant in IPA-infected individuals with COPD (0.81) than those with ILD (1.39) and NCFB (1.57, Table 3, Figure 1D). These findings corroborated the use of corticosteroid and broad-spectrum antibiotics, both of which were most common in individuals with COPD among all IPA patients (41/72, 57%, Table 2) (see previous note on this statistic). Thus, one mechanism how structural lung disease confers immune dysfunction may be through long-term treatment with corticosteroids and broad-spectrum antibiotics.
Among IPA patients with COPD, 89% used corticosteroids (23/26), as opposed to only 67% of IPA patients with ILD (10/15). Corticosteroid use is known to affect IPA incidence in COPD cases (5,6): receiving accumulated doses of corticosteroids (e.g., >700 mg of prednisone) during the 3 months preceding hospital admission has been shown to significantly increase the risk of IPA occurrence in COPD patients (14). Studies by He et al. have suggested that cumulative doses of corticosteroids exceeding 350 mg are highly related to IPA occurrence in COPD patients (14,15), and some reports have even evoked a plausible role for inhaled steroids in its emergence (16-18). It has also been shown that immune dysfunction and bacterial persistence in NCFB are associated with the use of anti-inflammatory agents, such as inhaled corticosteroids (19), and long-term antibiotic treatment (20,21).
We also found that 69% of the COPD group used broad-spectrum antibiotics (18/26) (Table 2), as opposed to 53% (8/15) of those with ILD and 86.7% (13/15) of those with NCFB (Table 2). Both the use of broad-spectrum antibiotics to treat an acute bacteria exacerbation within the 3 months before hospital admission and the prescription of three or more broad-spectrum antibiotics in hospital are significant predictors of IPA in patients with COPD (6,15). These risk factors led Bulpa et al. to propose an adapted version of IPA diagnostic criteria in 2007, standardizing the definitions of the non-immunocompromised population with a focus on COPD patients (22).
The impairment of host immune defense plays a major role in IPA pathogenesis. Airway macrophages ingest and destroy A. conidia cells, and germinating spores and hyphae are attacked by polymorphonuclear neutrophils (PMNs) (23). In COPD, although there is increased recruitment and activation of eosinophils, pulmonary alveolar macrophages (PAMs), and lymphocytes within the lungs, their immune functions of phagocytosis and chemotaxis are qualitatively altered (24,25). Evidence now shows that COPD progresses to systemic autoimmunity, possibly through spillover from the primary pulmonary inflammatory input (26-28). The recognition of pathogen-associated molecular patterns by host cell pattern recognition receptors (PRRs) is the first step of phagocytosis, leading to a pro-inflammatory response characterized by the production of cytokines and chemokines. Toll-like receptor 2 (TLR2) and 4 (TLR4) are among the PRRs most recognized by the body, and a decrease in their expression in COPD patients (29,30) could diminish the initial innate immune response against Aspergillus. Corticosteroids also contribute to the development of IPA by inhibiting the phagocytosis functions of PAMs and PMNs (31,32), and may also stimulate Aspergillus fumigatus(A. fumigatus) growth via action from a sterol-binding protein (33).
As shown in Table 3 and Figure 2, IgA levels were lowest in IPA patients with NCFB (0.95) compared to those with COPD (1.64) and ILD (3.16) (Table 3, Figure 2). Decreased sIgA levels lead to immunocompromisation and result in critical LRT infections that prompt the use of broad-spectrum antibiotics, which in turn bolsters fungal emergence in the airways (34). Pseudomonas aeruginosa (P. aeruginosa), a Gram-negative non-fermenting bacterium found in bronchiectasis patients with severely affected pulmonary function (e.g., requiring mechanical ventilation), is known for its mutational and biofilm producing abilities, conferring it resistance to antibiotics (35). As A. fumigatus is known to grow as a biofilm in vivo, the possibility of an interaction between these two species has been suggested, with P. aeruginosa providing biofilm niches that allow the expansion of invasive fungal colonies (36).
Conclusions
Despite the high mortality rate of IPA patients, the sensitivity of LRT cultures and serology remain poor. We show herein that cellular and humoral immunity parameters vary with the degree of damage induced by invasive pulmonary fungal infections when combined with structural lung disease, and establish a foundation for future inroads into IPA immunotherapy research. In-depth studies should be carried out to further assess IPA, with the aim of characterizing its genesis, occurrence, development, and effects on the immune system, thereby clarifying its immune pathogenesis and providing important theoretical bases for immune intervention. Immune status should be taken into account in the differential diagnosis of IPA, especially in patients with COPD and NCFB under treatment with broad-spectrum antibiotics and steroids.
Acknowledgements
The authors would like to thank Arthur Chang for his English-language revision.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee board of the Shanghai Pulmonary Hospital (No. K16-322) and was consistent with the principles outlined in the Declaration of Helsinki.
References
- Chakrabarti A, Chatterjee SS, Das A, et al. Invasive aspergillosis in developing countries. Med Mycol 2011;49:S35-S47. [Crossref] [PubMed]
- Marchetti O, Lamoth F, Mikulska M, et al. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant 2012;47:846-54. [Crossref] [PubMed]
- Meersseman W, Vandecasteele SJ, Wilmer A, et al. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med 2004;170:621-5. [Crossref] [PubMed]
- Ribaud P, Chastang C, Latgé JP, et al. Survival and prognostic factors of invasive aspergillosis after allogeneic bone marrow transplantation. Clin Infect Dis 1999;28:322-30. [Crossref] [PubMed]
- Bulpa P, Dive A, Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur Respir J 2007;30:782-800. [Crossref] [PubMed]
- Guinea J, Torres-Narbona M, Gijón P, et al. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: incidence, risk factors, and outcome. Clin Microbiol Infect 2010;16:870-7. [Crossref] [PubMed]
- Xu H, Li L, Huang WJ, et al. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: a case control study from China. Clin Microbiol Infect 2012;18:403-8. [Crossref] [PubMed]
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21. [Crossref] [PubMed]
- Desoubeaux G, Bailly É, Chandenier J. Diagnosis of invasive pulmonary aspergillosis: Updates and recommendations. Med Mal Infect 2014;44:89-101. [Crossref] [PubMed]
- Vestbo J, Hurd SS, Agustí AG, et al. Sin, and Roberto Rodriguez-Roisin. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [Crossref] [PubMed]
- Barker AF. Bronchiectasis. N Engl J Med 2002;346:1383-93. [Crossref] [PubMed]
- Cohen M, Sahn SA. Bronchiectasis in systemic disease. Chest 1999;116:1063-74. [Crossref] [PubMed]
- Meyer KC, Raghu G, Baughman RP, et al. An Official American Thoracic Society clinical practice guideline: The Clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004-14. [Crossref] [PubMed]
- He H, Ding L, Li F, et al. Clinical features of invasive bronchial-pulmonary aspergillosis in critically ill patients with chronic obstructive respiratory diseases: a prospective study. Crit Care 2011;15:R5. [Crossref] [PubMed]
- He HY, Chang S, Ding L, et al. Significance of Aspergillus spp. isolation from lower respiratory tract samples for the diagnosis and prognosis of invasive pulmonary aspergillosis in chronic obstructive pulmonary disease. Chin Med J (Engl) 2012;125:2973-8. [PubMed]
- Leav BA, Fanburg B, Hadley S. Invasive pulmonary aspergillosis associated with high-dose inhaled fluticasone. N Engl J Med 2000;343:586. [Crossref] [PubMed]
- Peter E, Bakri F, Ball DM, et al. Invasive pulmonary filamentous fungal infection in a patient receiving inhaled corticosteroid therapy. Clin Infect Dis 2002;35:e54-6. [Crossref] [PubMed]
- Barouky R, Badet M, Denis MS, et al. Inhaled corticosteroids in chronic obstructive pulmonary disease and disseminated aspergillosis. Eur J Intern Med 2003;14:380-2. [Crossref] [PubMed]
- Kapur N, Bell S, Kolbe J, et al. Inhaled steroids for bronchiectasis. Cochrane Database Syst Rev 2009.CD000996. [PubMed]
- Evans DJ, Bara AI, Greenstone M. Prolonged antibiotics for purulent bronchiectasis in children and adults. Cochrane Database Syst Rev 2007.CD001392. [PubMed]
- Tsang KW, Ho PI, Chan KN, et al. A pilot study of low-dose erythromycin in bronchiectasis. Eur Respir J 1999;13:361-4. [Crossref] [PubMed]
- Bulpa P, Dive A, Sibille Y. Invasive pulmonary aspergillosisin patients with chronic obstructive pulmonary disease. Eur Respir J 2007;30:782-800. [Crossref] [PubMed]
- Romani L. Immunity to fungal infections. Nat Rev Immunol 2004;4:1-23. [Crossref] [PubMed]
- Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J 2008;31:1334-56. [Crossref] [PubMed]
- Berenson CS, Wrona CT, Grove LJ, et al. Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease. Am J Respir Crit Care Med 2006;174:31-40. [Crossref] [PubMed]
- Balloy V, Sallenave JM, Wu Y, et al. Aspergillus fumigates-induced interleukin-8 synthesis by respiratory epithelial cells is controlled by the phosphatidylinositol 3-kinase, p38 MAPK, and ERK1/2 pathways and not by the toll-like receptor-MyD88 pathway. J Biol Chem 2008;283:30513-21. [Crossref] [PubMed]
- Bellanger AP, Millon L, Khoufache K, et al. Aspergillus fumigatus germ tube growth and not conidia ingestion induces expression of inflammatory mediator genes in the human lung epithelial cell line A549. J Med Microbiol 2009;58:174-9. [Crossref] [PubMed]
- Netea MG, Ferwerda G, van der Graaf CA, et al. Recognition of fungal pathogens by toll-like receptors. Curr Pharm Des 2006;12:4195-201. [Crossref] [PubMed]
- MacRedmond RE, Greene CM, Dorscheid DR, et al. Epithelial expression of TLR4 is modulated in COPD and by steroids, salmeterol and cigarette smoke. Respir Res 2007;8:84. [Crossref] [PubMed]
- Droemann D, Goldmann T, Tiedje T, et al. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res 2005;6:68. [Crossref] [PubMed]
- Schaffner A. Therapeutic concentrations of glucocorticoids suppress the antimicrobial activity of human macrophages without impairing their responsiveness to gamma interferon. J Clin Invest 1985;76:1755-64. [Crossref] [PubMed]
- Diamond RD. Inhibition of monocyte-mediated damage to fungal hyphae by steroid hormones. J Infect Dis 1983;147:160. [Crossref] [PubMed]
- Ng TT, Robson GD, Denning DW. Hydrocortisone-enhanced growth of Aspergillus spp.: implications for pathogenesis. Microbiology 1994;140:2475-9. [Crossref] [PubMed]
- Chalmers JD., Hill AT. Mechanisms of immune dysfunction and bacterial persistence in non-cystic fibrosis bronchiectasis. Mol Immunol 2013;55:27-34. [Crossref] [PubMed]
- Martínez-Solano L, Macia MD, Fajardo A, et al. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin Infect Dis 2008;47:1526-33. [Crossref] [PubMed]
- Seidler MJ, Salvenmoser S, Müller FM. Aspergillus fumigates forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob Agents Chemother 2008;52:4130-6. [Crossref] [PubMed]


