Early management of sepsis with emphasis on early goal directed therapy: AME evidence series 002
Introduction
The Sepsis-3.0 consensus defined sepsis as “life-threatening organ dysfunction caused by dysregulated host response to infection” and it is clinically determined based on sequential organ dysfunction assessment (SOFA) score ≥2 points (1). The term “sepsis” in Sepsis-3.0 corresponds to the term “severe sepsis” in 2012 SSC guidelines (2). However, Sepsis-3.0 has not been fully validated and most studies included in this review employed the term “severe sepsis”. In the review, we adopted the term “severe sepsis” to make it consistent with existing literature. There is general consensus that early recognition and timely treatment largely determine outcome of sepsis and septic shock (3-5). Early recognition refers to the prompt identification of patients presenting with an acute systemic inflammatory response to infection. Depending on sepsis onset, this may occur in the emergency department (ED), ICU, general ward or even during the pre-hospital phase (6). Sepsis unleashes various heterogeneous systemic reactions which interfere with many physiological pathways to finally assault and harm organs. Except for hemodynamic and organ support, there is no “ready-made” treatment for sepsis. Current sepsis management therefore focuses primarily on early goal directed therapy (EGDT) and/or bundle treatment. EGDT promotes prompt recognition of sepsis followed by starting up a treatment “bundle” within the first 3 to 6 hours following diagnosis. Because of methodological issues, several recent large multicenter studies failed to demonstrate a beneficial effect of EGDT as compared to usual care. Yet, the conceptual framework underlying EGDT is still considered as the cornerstone for the early management of sepsis and septic shock (7,8).
Early goal directed therapy (EGDT)
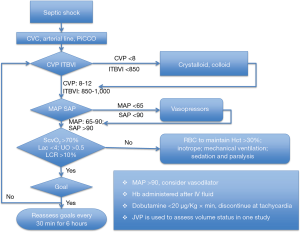
Clinical application of an EGDT protocol was first reported in a single-center study, recruiting patients on arrival at the ED (3). Compared with standard care, EGDT decreased mortality rate by 16%. Basically, EGDT aimed to obtain distinct resuscitation goals [i.e., central venous pressure (CVP) =8–12 mmHg; mean arterial pressure (MAP) =65–90 mmHg; urinary output >0.5 mL/kg/hour; central venous oxygen saturation (ScvO2) >70% within the first six hours] (Figure 1). The EGDT protocol comprised infusion of colloids and crystalloid fluids to increase effective circulatory volume, vasopressor administration to raise MAP and, as needed, blood cell transfusion, inotropes, mechanical ventilation or curarization to ensure a correct balance between oxygen supply and consumption. During the following years, the EGDT concept was progressively expanded to incorporate interventions such as early initiation of antibiotics, adequate source control, and more elaborated fluid and hemodynamic resuscitation measures. EGDT was also the subject of both experimental and observational studies and became a widely accepted treatment approach. The Surviving Sepsis Campaign (SSC) guidelines proclaimed some components of EGDT as the standard treatment for patients with sepsis and septic shock (2,9). However, the beneficial effect of EGDT has been challenged by several large trials (10,11), which will be discussed in the following sections.
Literature search
We conducted a systematic literature review to investigate the efficacy of EGDT on patient-important outcomes. Only original studies involving human subjects were included. An electronic PubMed search was performed using the following strategy and key words: (((((sepsis[Title/Abstract]) OR septic[Title/Abstract]) OR bacteremia[Title/Abstract]) AND Clinical Trial[ptyp])) AND (((early goal[Title/Abstract]) OR goal directed therapy[Title/Abstract]) AND Clinical Trial[ptyp]).
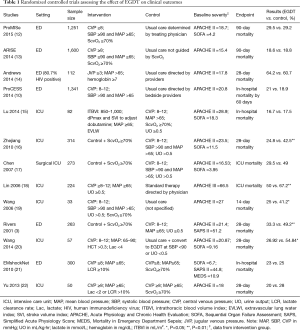
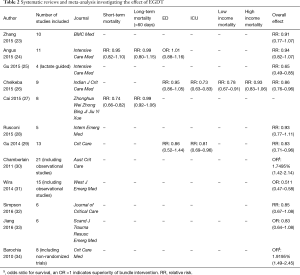
The initial search yielded 38 citations. Additional screening identified ten randomized controlled trials (RCTs) that specifically investigated the efficacy of EGDT (Table 1). Manual review of the references accompanying these publications detected three more studies that fulfilled screening criteria. Finally, 13 RCTs (Table 1) and 12 systematic reviews and meta-analyses on EGDT were identified for the review (Table 2).
Full table
Full table
Main findings
EGDT was found to significantly benefit mortality as compared with standard care in 5 of the 13 studies (3,15,17,27,35). Of these, four were performed in China and one was the Rivers seminal study. There was significant heterogeneity among included RCTs and subgroup analysis showed that the heterogeneity could be explained by some factors (I2=64%, P=0.02). For example, the beneficial effect of EGDT was influenced by the economic status of the involved research centers and only confirmed in studies from low-income countries [RR: 0.78 (0.67–0.91) vs. 0.93 (0.83–1.06) for low- and high-income countries respectively] (26). A trial by Chen et al. was conducted in a population with lower socio-economic status and less access to hospital care, which reported significantly lower mortality rate in the treatment group (29.5% vs. 49.0%; P=0.007) (17). However, the study by Andrews et al. was also performed in low-income country, but it reported higher mortality rate in the EGDT group (64.2% vs. 60.7%; P>0.05). Thus, the impact of economic status on the effect of EGDT remains inconclusive.
Another explanation for the heterogeneity among RCTs is the baseline mortality risk of enrolled subjects. Since the first introduction of EGDT by Rivers, its implementation in clinical practice had markedly improved management of sepsis and probably explained the significant reduction in mortality over the last ten years (Table 1) (36-38). Control patients in the three recently published large clinical trials (ProMISe, ARISE and ProCESS, also called “a trio of trials”) literally received “standard” care, but might well have been treated according to EGDT principles (8,10,12,13). Note that EGDT also implies extensive “bundled” treatments including immediate fluid resuscitation, early and adequate source control and/or initiation of antibiotics, and tailored use of vasopressors, inotropes and blood transfusion (39). Although study protocols dictated that the control group was not guided by ScvO2 monitoring but directed by the treating physician, many components of the SSC bundle could have been introduced (35,40), which may explain why no significant difference was found between standard and EGDT-driven care. As an example, baseline ScvO2 in these studies was higher than 70% in most of patients, which may reflect an aggressive medical therapy before randomization or the inclusion of less severe patients than in the Rivers study. Measuring lactate clearance is as efficient as ScvO2 (41), and the availability of lactate clearance in the control arm results in similar outcomes between the two groups.
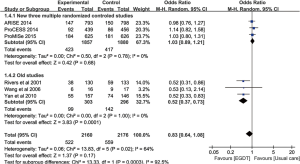
However, only the Rivers study reported a highly significant beneficial effect of EGDT on patient outcome. Importantly, mortality rate in the Rivers’ control population (49.2%) was much higher than in the ProMISe (29.2%), ARISE (18.8%) and ProCESS (18.9%) controls. It is conceivable that a potential EGDT effect was confounded by this low baseline mortality. Jiang and colleagues showed that the beneficial effects of EGDT were only observed in the oldest studies (RR: 0.52; 95% CI: 0.37–0.73), which support the notion that the control group in recent trials had probably been treated in accordance with EGDT precepts (Figure 2) (33). Interestingly, Simpson and colleagues employed meta-regression analysis to investigate the effect of control group mortality, initial APACHE score, year of publication, and use of central venous catheters in the usual care group on heterogeneity of trial outcomes reported in all meta-analyses of EGDT. They found that the baseline or control group mortality rate explains a significant portion of the heterogeneity (R2 =0.57; P=0.042) (32).
Interestingly, observational studies generally reported better efficacy of EGDT than RCTs (40,42-50). Three of the ten systematic review and meta-analyses reported evidence of significant EGDT benefit (30,31,34), yet were probably biased by including observational studies. Even adjustment for confounders may not exclude the impact of numerous unmeasured factors on the results. In contrast to RCTs, observational studies tend to overestimate treatment effects (51). Also, observational studies mostly are explicitly or implicitly data- rather than hypothesis-driven. Statistical analysis is not predefined but performed after reviewing the data which may lead to multiple testing and selective reporting (52). Furthermore, the conduct of studies cannot be explicitly monitored with validated methodology. Thus, observational studies offer preliminary information with low level of evidence. Others argue that RCTs impose the Hawthorne effect that the control group is improved as compared with normal clinical circumstances (53).
EGDT setting
In the Rivers study, septic shock was regarded as an emergency department study and EGDT was applied immediately after ED admission. The care provided included the use of lactate and ScvO2, which were completely blinded to the ICU clinicians. The three most recent RCTs (ProMISe, ARISE and ProCESS) were not blinded as the patients were in the ICU within 2 hours of presentation and the longitudinal care of using lactate and ScvO2 was not blinded (10,12,13). It is possible that the EGDT effect can be diminished by the lack of blinding and the use of numerous principles of EGDT in all treatment groups (8).
In contrast, several Chinese studies in ICU patients all reported better survival in EGDT receivers (16-20). EGDT practice in ICU is conceptually less attractive because substantial time may be lost between arrival in the ED and transfer to the ICU. The critical 3- to 6-hour resuscitation window may be neglected or inadvertently allowed to pass. However, timely EGDT can be performed in patients who enter the ICU directly from the hospital ward or operating room. Based on our experience, a mixed ICU receives half of patients from the ED and the other half from the ward and operating theatre (54). Unfortunately, EGDT studies did not report the time from onset of septic shock to ED and/or ICU admission, because it was difficult or even impossible to determine (24,26,29). Thus, the benefit of EGDT performed in the ED can be confounded by the time between disease onset and ED arrival. In other words, the beneficial effect of EGDT can be diminished by delayed ED arrival and initiation of systemic treatment. Postsurgical patients and patients entering the ICU from hospital wards may benefit more from promptly applying EGDT. Moreover, more accurate appreciation of the time span between sepsis onset and ICU admission provides room for improvement and assessment of EGDT practice in this particular population. However, the impact of timing of EGDT on mortality remains speculative. Preliminary evidence demonstrates that mortality reduction has been observed even with significant delays (up to 12 hours) in initiating EGDT (55-58).
Compliance with Surviving Sepsis Campaign (SSC) bundle
Unlike the negative results from recently published RCTs, most observational studies demonstrated that SSC bundle adherence was associated with a reduced mortality rate (9,59-74), but without significantly increasing medical cost (75). Over a 7.5-year period running from 2005 till 2012, Levy et al. found that compliance to the SSC bundle resulted in a 25% relative mortality risk reduction (76). For less “compliant” sites, hospital mortality rates dropped 0.7% per site for every three months of participation in an educational program (77). Other observational and quasi-experimental studies reported similar results (40,55,78-92). When EGDT was incorporated into a clinical pathway for the treatment of severe sepsis and septic shock, hospital mortality could be decreased (93). Due to the importance of adherence to EGDT bundle, studies investigated factors associated with compliance (94,95). These studies showed that body temperature, experience of nurses and physicians, cryptic shock, serum lactate levels and ED crowding all affected adherence (94,96).
An important distinction between RCTs exploring EGDT and observational studies applying the SSC bundle is that the latter included somewhat different components than recommended in EGDT. Another important difference lies in the management of control groups. Over time, the level of care in the control groups has probably been upgraded, introducing a Hawthorne effect. Taken together, the management of these patients perhaps does not reflect usual clinical practices, resulting in large difference between observational studies and RCTs. In fact, the SSC bundle not only underscores early use of antibiotics, obtaining blood cultures and measuring lactate, and setting resuscitation goals at CVP >8 mmHg, MAP >65 mmHg and ScvO2 >70%, but also included lung protective ventilation, administration of steroids, tight glucose control and, initially, adjunctive treatment with drotrecogin alfa (a drug that is no longer marketed) (79). The SSC bundle thus represents a frame that allows comprehensive measures to act in concert to enhance the positive effect of each individual component. The effect of a single intervention could indeed become easily corrupted by a low signal-to-noise ratio precluding any valuable statistical interpretation (97). Importantly, mortality of sepsis and septic shock is markedly reduced when physicians are engaged in an educational and quality improvement programs that incorporates all systemic components recommended by the SCC guideline (91,98-100). High-level evidence of the effectiveness of the SSC bundle can only be obtained by conducting a RCT. However, such trial defies ethical standards since it would potentially deprive patients in the control group of evidence-based care. Yet, based on the consistently positive results and the large effect size (OR: 0.66, 95% CI: 0.61–0.72; k=48, N=434,447) reported in observational studies (79), implementation of the SSC bundle to treat sepsis and septic shock is highly recommended.
The importance of early recognition of sepsis
In some studies, delayed initiation of EGDT was associated with improved outcome, compared with non-compliance, suggesting that late initiation is better than no initiation (56-58). However, these studies included initiation of EGDT more than 12 hours after diagnosis in the definition of non-compliance. Delays of this magnitude have been associated with increased risk of death (101). Another study showed that inability to achieve early resuscitation goals (OR: 1.94, 95% CI: 1.0–3.51) was associated with increased 28-day mortality rate (102). Thus, prompt initiation of EGDT is still recommended for septic shock (103-108), and many efforts have been made to improve early recognition and treatment of septic shock (109). However, early recognition of severe sepsis and septic shock, albeit crucial for EGDT efficacy, remains elusive in clinical practice. For example, the Rivers’ study criteria dictated that EGDT maneuvers had to be completed within 6 hours after ED admission (3). However, this 6-hour time window obviously could not have been the same to all patients, who were septic for an unspecified time period before ED admission. Tools that reliably detect early community-acquired sepsis are warranted and are the topic of intensive research. Meanwhile, there are studies focusing on early recognition of sepsis in hospitalized patients (110-112). As mentioned before, this patient population may be identified more easily and thus become more amenable for straightforward EGDT. The SEPSIS KILL program aimed to provide interventions within 60 minutes after onset of sepsis in hospitalized patients, which was shown to be effective in reducing mortality (113).
Studies investigating the impact of automated electronic sepsis alert systems on clinical outcome produced inconsistent results (Table 3) (112). Two RCTs failed to show a beneficial effect of such alert systems (114,118). In contrast, a before-after study showed that applying an alert system resulted in a decreased mortality (117). Time to completion of the resuscitation bundle was not influenced by the use of an electronic alert system (118). Of note is that SIRS criteria, which are challenged for lack of specificity, were mainly used as alerting threshold (120). A new definition of sepsis (Sepsis-3), incorporating an adapted organ dysfunction (quick SOFA) score to identify sepsis in an early phase has recently been released (1,121). The consensus conference recommended that the quick SOFA that includes altered mental status, fast respiratory rate and low blood pressure should be widely diffused in order to improve the early detection of sepsis. More recently, Churpek and colleagues demonstrated that both the Modified Early Warning System (MEWS) and the National Early Warning System (NEWS) demonstrate higher predictive ability for mortality or prolonged ICU stay than qSOFA (122). Trials to investigate whether electronic alerting systems based on Sepsis-3 or other measures will fine-tune sepsis management and improve relevant patient-important outcomes are awaited. Recognition of sepsis with automated sepsis alert systems is not necessarily coupled with initiating the SSC bundle, so it remains to be proven whether triggering SSC bundle therapy by automated sepsis alert systems improves clinical outcomes.
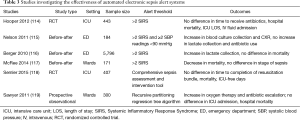
Full table
Conclusions
Sepsis is a heterogeneous syndrome and thus unlikely to respond to a single treatment. Within this context, EGDT has been introduced as an interesting approach characterized by early recognition and prompt initiation of a structured treatment algorithm. Initially, EGDT targeted a CVP >8 mmHg, a MAP >65 mmHg, a diuresis >0.5 mL/kg/hour and a ScvO2 >70%. Thereafter, EGDT was progressively expanded by adding lactate (clearance) as a supplementary goal and including interventions such as early and adequate source control and/or antibiotic use, low tidal volume mechanical ventilation, and steroid administration (123). Such expanded EGDT algorithm is incorporated in the SSC bundle. Current evidence supports the idea that EGDT may benefit ICU patients more than ED subjects because of a better knowledge of the time window between diagnosis of sepsis and start of treatment. Several observational studies have reported a beneficial effect of SSC bundle adherence on patient-important outcomes but this was not confirmed by recent large RCTs. This is due to a progressive decrease of mortality over time in patients receiving “standard” care which probably implicitly implies more adequate resuscitation. Taken together, early awareness of sepsis and upfront initiation of the SSC bundle remain imperative for improving the fate of severely septic patients.
Acknowledgements
We would like to thank Prof. Emanuel Rivers (Department of Emergency Medicine, Henry Ford Hospital, Wayne State University, Detroit, MI, USA) for his invaluable suggestions on this manuscript drafting.
Funding: The study was supported by Zhejiang Medical Science and Technology projects (2015117919) and Zhejiang Provincial Science and technology projects (2013C33G2010401). The funder has no role in the design and conduct of the review.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, Ressler J, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med 2007;35:1105-12. [Crossref] [PubMed]
- Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010;38:1045-53. [Crossref] [PubMed]
- Yealy DM, Huang DT, Delaney A, et al. Recognizing and managing sepsis: what needs to be done? BMC Med 2015;13:98. [Crossref] [PubMed]
- Head LW, Coopersmith CM. Evolution of Sepsis Management: From Early Goal-Directed Therapy to Personalized Care. Adv Surg 2016;50:221-34. [Crossref] [PubMed]
- Nguyen HB, Jaehne AK, Jayaprakash N, et al. Early goal-directed therapy in severe sepsis and septic shock: insights and comparisons to ProCESS, ProMISe, and ARISE. Crit Care 2016;20:160. [Crossref] [PubMed]
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010;38:367-74. [Crossref] [PubMed]
- ProCESS Investigators., Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683-93. [Crossref] [PubMed]
- Gupta RG, Hartigan SM, Kashiouris MG, et al. Early goal-directed resuscitation of patients with septic shock: current evidence and future directions. Crit Care 2015;19:286. [Crossref] [PubMed]
- Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015;372:1301-11. [Crossref] [PubMed]
- ARISE Investigators.; ANZICS Clinical Trials Group., Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496-506. [Crossref] [PubMed]
- Andrews B, Muchemwa L, Kelly P, et al. Simplified severe sepsis protocol: a randomized controlled trial of modified early goal-directed therapy in Zambia. Crit Care Med 2014;42:2315-24. [Crossref] [PubMed]
- Lu N, Zheng R, Lin H, et al. Clinical studies of surviving sepsis bundles according to PiCCO on septic shock patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014;26:23-7. [PubMed]
- Early Goal-Directed Therapy Collaborative Group of Zhejiang Province. The effect of early goal-directed therapy on treatment of critical patients with severe sepsis/septic shock: a multi-center, prospective, randomized, controlled study. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2010;22:331-4. [PubMed]
- Chen ZQ, Jin YH, Chen H, et al. Early goal-directed therapy lowers the incidence, severity and mortality of multiple organ dysfunction syndrome. Nan Fang Yi Ke Da Xue Xue Bao 2007;27:1892-5. [PubMed]
- Lin SM, Huang CD, Lin HC, et al. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock 2006;26:551-7. [Crossref] [PubMed]
- Wang XZ, Lü CJ, Gao FQ, et al. Efficacy of goal-directed therapy in the treatment of septic shock. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2006;18:661-4. [PubMed]
- Wang T, Xia Y, Hao D, et al. The significance of lactic acid in early diagnosis and goal-directed therapy of septic shock patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014;26:51-5. [PubMed]
- Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 2010;303:739-46. [Crossref] [PubMed]
- Yu B, Tian HY, Hu ZJ, et al. Comparison of the effect of fluid resuscitation as guided either by lactate clearance rate or by central venous oxygen saturation in patients with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2013;25:578-83. [PubMed]
- Zhang L, Zhu G, Han L, et al. Early goal-directed therapy in the management of severe sepsis or septic shock in adults: a meta-analysis of randomized controlled trials. BMC Med 2015;13:71. [Crossref] [PubMed]
- Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015;41:1549-60. [Crossref] [PubMed]
- Gu WJ, Zhang Z, Bakker J. Early lactate clearance-guided therapy in patients with sepsis: a meta-analysis with trial sequential analysis of randomized controlled trials. Intensive Care Med 2015;41:1862-3. [Crossref] [PubMed]
- Chelkeba L, Ahmadi A, Abdollahi M, et al. Early goal-directed therapy reduces mortality in adult patients with severe sepsis and septic shock: Systematic review and meta-analysis. Indian J Crit Care Med 2015;19:401-11. [Crossref] [PubMed]
- Cai G, Tong H, Hao X, et al. The effects of early goal-directed therapy on mortality rate in patients with severe sepsis and septic shock: a systematic literature review and Meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015;27:439-42. [PubMed]
- Rusconi AM, Bossi I, Lampard JG, et al. Early goal-directed therapy vs usual care in the treatment of severe sepsis and septic shock: a systematic review and meta-analysis. Intern Emerg Med 2015;10:731-43. [Crossref] [PubMed]
- Gu WJ, Wang F, Bakker J, et al. The effect of goal-directed therapy on mortality in patients with sepsis - earlier is better: a meta-analysis of randomized controlled trials. Crit Care 2014;18:570. [Crossref] [PubMed]
- Chamberlain DJ, Willis EM, Bersten AB. The severe sepsis bundles as processes of care: a meta-analysis. Aust Crit Care 2011;24:229-43. [Crossref] [PubMed]
- Wira CR, Dodge K, Sather J, et al. Meta-analysis of protocolized goal-directed hemodynamic optimization for the management of severe sepsis and septic shock in the Emergency Department. West J Emerg Med 2014;15:51-9. [Crossref] [PubMed]
- Simpson SQ, Gaines M, Hussein Y, et al. Early goal-directed therapy for severe sepsis and septic shock: A living systematic review. J Crit Care 2016;36:43-8. [Crossref] [PubMed]
- Jiang LB, Zhang M, Jiang SY, et al. Early goal-directed resuscitation for patients with severe sepsis and septic shock: a meta-analysis and trial sequential analysis. Scand J Trauma Resusc Emerg Med 2016;24:23. [Crossref] [PubMed]
- Barochia AV, Cui X, Vitberg D, et al. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med 2010;38:668-78. [Crossref] [PubMed]
- Liu B, Ding X, Yang J. Effect of early goal directed therapy in the treatment of severe sepsis and/or septic shock. Curr Med Res Opin 2016;32:1773-82. [Crossref] [PubMed]
- Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med 2016;193:259-72. [Crossref] [PubMed]
- Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308-16. [Crossref] [PubMed]
- Schlapbach LJ, Straney L, Alexander J, et al. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002-13: a multicentre retrospective cohort study. Lancet Infect Dis 2015;15:46-54. [Crossref] [PubMed]
- Shapiro NI, Howell MD, Talmor D, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med 2006;34:1025-32. [Crossref] [PubMed]
- Ferrer R, Artigas A, Levy MM, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA 2008;299:2294-303. [Crossref] [PubMed]
- Jansen TC, van Bommel J, Schoonderbeek FJ, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med 2010;182:752-61. [Crossref] [PubMed]
- Apibunyopas Y. Mortality rate among patients with septic shock after implementation of 6-hour sepsis protocol in the emergency department of Thammasat University Hospital. J Med Assoc Thai 2014;97 Suppl 8:S182-93. [PubMed]
- Cannon CM, Holthaus CV, Zubrow MT, et al. The GENESIS project (GENeralized Early Sepsis Intervention Strategies): a multicenter quality improvement collaborative. J Intensive Care Med 2013;28:355-68. [Crossref] [PubMed]
- Cardoso T, Carneiro AH, Ribeiro O, et al. Reducing mortality in severe sepsis with the implementation of a core 6-hour bundle: results from the Portuguese community-acquired sepsis study (SACiUCI study). Crit Care 2010;14:R83. [Crossref] [PubMed]
- Casserly B, Baram M, Walsh P, et al. Implementing a collaborative protocol in a sepsis intervention program: lessons learned. Lung 2011;189:11-9. [Crossref] [PubMed]
- Focht A, Jones AE, Lowe TJ. Early goal-directed therapy: improving mortality and morbidity of sepsis in the emergency department. Jt Comm J Qual Patient Saf 2009;35:186-91. [Crossref] [PubMed]
- Gurnani PK, Patel GP, Crank CW, et al. Impact of the implementation of a sepsis protocol for the management of fluid-refractory septic shock: A single-center, before-and-after study. Clin Ther 2010;32:1285-93. [Crossref] [PubMed]
- Jacob ST, Banura P, Baeten JM, et al. The impact of early monitored management on survival in hospitalized adult Ugandan patients with severe sepsis: a prospective intervention study*. Crit Care Med 2012;40:2050-8. [Crossref] [PubMed]
- Jones AE, Focht A, Horton JM, et al. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest 2007;132:425-32. [Crossref] [PubMed]
- Jones AE, Troyer JL, Kline JA. Cost-effectiveness of an emergency department-based early sepsis resuscitation protocol. Crit Care Med 2011;39:1306-12. [Crossref] [PubMed]
- Zhang Z, Ni H, Xu X. Do the observational studies using propensity score analysis agree with randomized controlled trials in the area of sepsis? J Crit Care 2014;29:886.e9-15. [Crossref] [PubMed]
- Norris SL, Moher D, Reeves BC, et al. Issues relating to selective reporting when including non-randomized studies in systematic reviews on the effects of healthcare interventions. Res Synth Methods 2013;4:36-47. [Crossref] [PubMed]
- Manek NJ, Tiller WA. A new perspective on “the placebo effect”: untangling the entanglement. Med Hypotheses 2011;77:614-9. [Crossref] [PubMed]
- Zhang Z, Ni H, Qian Z. Effectiveness of treatment based on PiCCO parameters in critically ill patients with septic shock and/or acute respiratory distress syndrome: a randomized controlled trial. Intensive Care Med 2015;41:444-51. [Crossref] [PubMed]
- Castellanos-Ortega A, Suberviola B, García-Astudillo LA, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med 2010;38:1036-43. [Crossref] [PubMed]
- Gao F, Melody T, Daniels DF, et al. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study. Crit Care 2005;9:R764-70. [Crossref] [PubMed]
- Coba V, Whitmill M, Mooney R, et al. Resuscitation bundle compliance in severe sepsis and septic shock: improves survival, is better late than never. J Intensive Care Med 2011;26:304-13. [Crossref] [PubMed]
- Castellanos-Ortega Á, Suberviola B, García-Astudillo LA, et al. Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock 2011;36:542-7. [Crossref] [PubMed]
- Guo Q, Li HY, Li YM, et al. Compliance with severe sepsis bundles and its effect on patient outcomes of severe community-acquired pneumonia in a limited resources country. Arch Med Sci 2014;10:970-8. [Crossref] [PubMed]
- Kuan WS, Mahadevan M, Tan JH, et al. Feasibility of introduction and implementation of the Surviving Sepsis Campaign bundle in a Singapore emergency department. Eur J Emerg Med 2013;20:344-9. [Crossref] [PubMed]
- Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med 2006;34:2707-13. [Crossref] [PubMed]
- Miller RR, Dong L, Nelson NC, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med 2013;188:77-82. [Crossref] [PubMed]
- Na S, Kuan WS, Mahadevan M, et al. Implementation of early goal-directed therapy and the surviving sepsis campaign resuscitation bundle in Asia. Int J Qual Health Care 2012;24:452-62. [Crossref] [PubMed]
- Noritomi DT, Ranzani OT, Monteiro MB, et al. Implementation of a multifaceted sepsis education program in an emerging country setting: clinical outcomes and cost-effectiveness in a long-term follow-up study. Intensive Care Med 2014;40:182-91. [Crossref] [PubMed]
- Qu HP, Qin S, Min D, et al. The effects of earlier resuscitation on following therapeutic response in sepsis with hypoperfusion. Zhonghua Wai Ke Za Zhi 2006;44:1193-6. [PubMed]
- Rinaldi L, Ferrari E, Marietta M, et al. Effectiveness of sepsis bundle application in cirrhotic patients with septic shock: a single-center experience. J Crit Care 2013;28:152-7. [Crossref] [PubMed]
- Schramm GE, Kashyap R, Mullon JJ, et al. Septic shock: a multidisciplinary response team and weekly feedback to clinicians improve the process of care and mortality. Crit Care Med 2011;39:252-8. [Crossref] [PubMed]
- Wang Z, Xiong Y, Schorr C, et al. Impact of sepsis bundle strategy on outcomes of patients suffering from severe sepsis and septic shock in china. J Emerg Med 2013;44:735-41. [Crossref] [PubMed]
- Worapratya P, Wanjaroenchaisuk A, Joraluck J, et al. Success of applying early goal-directed therapy for septic shock patients in the emergency department. Open Access Emerg Med 2016;8:1-6. [Crossref] [PubMed]
- Thompson MP, Reeves MJ, Bogan BL, et al. Protocol-Based Resuscitation Bundle to Improve Outcomes in Septic Shock Patients: Evaluation of the Michigan Health and Hospital Association Keystone Sepsis Collaborative. Crit Care Med 2016;44:2123-30. [Crossref] [PubMed]
- McColl T, Gatien M, Calder L, et al. Implementation of an Emergency Department Sepsis Bundle and System Redesign: A Process Improvement Initiative. CJEM 2016.1-10. [Epub ahead of print]. [Crossref] [PubMed]
- El Solh AA, Akinnusi ME, Alsawalha LN, et al. Outcome of septic shock in older adults after implementation of the sepsis "bundle". J Am Geriatr Soc 2008;56:272-8. [Crossref] [PubMed]
- Lefrant JY, Muller L, Raillard A, et al. Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: a multicenter study. Ann Fr Anesth Reanim 2010;29:621-8. [Crossref] [PubMed]
- Sivayoham N, Rhodes A, Jaiganesh T, et al. Outcomes from implementing early goal-directed therapy for severe sepsis and septic shock: a 4-year observational cohort study. Eur J Emerg Med 2012;19:235-40. [Crossref] [PubMed]
- Assuncao MS, Teich V, Shiramizo SC, et al. The cost-effectiveness ratio of a managed protocol for severe sepsis. J Crit Care 2014;29:692.e1-6. [Crossref] [PubMed]
- Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015;43:3-12. [Crossref] [PubMed]
- Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med 2014;40:1623-33. [Crossref] [PubMed]
- Rhodes A, Phillips G, Beale R, et al. The Surviving Sepsis Campaign bundles and outcome: results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study). Intensive Care Med 2015;41:1620-8. [Crossref] [PubMed]
- Damiani E, Donati A, Serafini G, et al. Effect of performance improvement programs on compliance with sepsis bundles and mortality: a systematic review and meta-analysis of observational studies. PLoS One 2015;10:e0125827. [Crossref] [PubMed]
- van Zanten AR, Brinkman S, Arbous MS, et al. Guideline bundles adherence and mortality in severe sepsis and septic shock. Crit Care Med 2014;42:1890-8. [Crossref] [PubMed]
- De Miguel-Yanes JM, Muñoz-González J, Andueza-Lillo JA, et al. Implementation of a bundle of actions to improve adherence to the Surviving Sepsis Campaign guidelines at the ED. Am J Emerg Med 2009;27:668-74. [Crossref] [PubMed]
- MacRedmond R, Hollohan K, Stenstrom R, et al. Introduction of a comprehensive management protocol for severe sepsis is associated with sustained improvements in timeliness of care and survival. Qual Saf Health Care 2010;19:e46. [PubMed]
- McKinley BA, Moore LJ, Sucher JF, et al. Computer protocol facilitates evidence-based care of sepsis in the surgical intensive care unit. J Trauma 2011;70:1153-66; discussion 1166-7. [Crossref] [PubMed]
- Siontis B, Elmer J, Dannielson R, et al. Multifaceted interventions to decrease mortality in patients with severe sepsis/septic shock-a quality improvement project. PeerJ 2015;3:e1290. [Crossref] [PubMed]
- Patel GW, Roderman N, Gehring H, et al. Assessing the effect of the Surviving Sepsis Campaign treatment guidelines on clinical outcomes in a community hospital. Ann Pharmacother 2010;44:1733-8. [Crossref] [PubMed]
- Puskarich MA, Marchick MR, Kline JA, et al. One year mortality of patients treated with an emergency department based early goal directed therapy protocol for severe sepsis and septic shock: a before and after study. Crit Care 2009;13:R167. [Crossref] [PubMed]
- Memon JI, Rehmani RS, Alaithan AM, et al. Impact of 6-hour sepsis resuscitation bundle compliance on hospital mortality in a saudi hospital. Crit Care Res Pract 2012;2012:273268.
- Shiramizo SC, Marra AR, Durão MS, et al. Decreasing mortality in severe sepsis and septic shock patients by implementing a sepsis bundle in a hospital setting. PLoS One 2011;6:e26790. [Crossref] [PubMed]
- Hanzelka KM, Yeung SC, Chisholm G, et al. Implementation of modified early-goal directed therapy for sepsis in the emergency center of a comprehensive cancer center. Support Care Cancer 2013;21:727-34. [Crossref] [PubMed]
- Kortgen A, Niederprüm P, Bauer M. Implementation of an evidence-based “standard operating procedure” and outcome in septic shock. Crit Care Med 2006;34:943-9. [Crossref] [PubMed]
- Thiel SW, Asghar MF, Micek ST, et al. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med 2009;37:819-24. [Crossref] [PubMed]
- Carvas JM, Canelas C, Montanha G, et al. Impact of Compliance with a Sepsis Resuscitation Bundle in a Portuguese Emergency Department. Acta Med Port 2016;29:88-94. [Crossref] [PubMed]
- Laguna-Pérez A, Chilet-Rosell E, Delgado Lacosta M, et al. Clinical pathway intervention compliance and effectiveness when used in the treatment of patients with severe sepsis and septic shock at an Intensive Care Unit in Spain. Rev Lat Am Enfermagem 2012;20:635-43. [Crossref] [PubMed]
- Kang MJ, Shin TG, Jo IJ, et al. Factors influencing compliance with early resuscitation bundle in the management of severe sepsis and septic shock. Shock 2012;38:474-9. [Crossref] [PubMed]
- Mikkelsen ME, Gaieski DF, Goyal M, et al. Factors associated with nonadherence to early goal-directed therapy in the ED. Chest 2010;138:551-8. [Crossref] [PubMed]
- Shin TG, Jo IJ, Choi DJ, et al. The adverse effect of emergency department crowding on compliance with the resuscitation bundle in the management of severe sepsis and septic shock. Crit Care 2013;17:R224. [Crossref] [PubMed]
- Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. Lancet Infect Dis 2015;15:581-614. [Crossref] [PubMed]
- Li CH, Kuan WS, Mahadevan M, et al. A multinational randomised study comparing didactic lectures with case scenario in a severe sepsis medical simulation course. Emerg Med J 2012;29:559-64. [Crossref] [PubMed]
- Tromp M, Bleeker-Rovers CP, van Achterberg T, et al. Internal medicine residents' knowledge about sepsis: effects of a teaching intervention. Neth J Med 2009;67:312-5. [PubMed]
- Trzeciak S, Dellinger RP, Abate NL, et al. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest 2006;129:225-32. [Crossref] [PubMed]
- Sohn CH, Ryoo SM, Seo DW, et al. Outcome of delayed resuscitation bundle achievement in emergency department patients with septic shock. Intern Emerg Med 2014;9:671-6. [Crossref] [PubMed]
- Ryoo SM, Kim WY, Sohn CH, et al. Prognostic value of timing of antibiotic administration in patients with septic shock treated with early quantitative resuscitation. Am J Med Sci 2015;349:328-33. [Crossref] [PubMed]
- Guerra WF, Mayfield TR, Meyers MS, et al. Early detection and treatment of patients with severe sepsis by prehospital personnel. J Emerg Med 2013;44:1116-25. [Crossref] [PubMed]
- Kliger J, Singer SJ, Hoffman FH. Using the integrated nurse leadership program to reduce sepsis mortality. Jt Comm J Qual Patient Saf 2015;41:264-72. [Crossref] [PubMed]
- Westphal GA, Koenig Á, Caldeira Filho M, et al. Reduced mortality after the implementation of a protocol for the early detection of severe sepsis. J Crit Care 2011;26:76-81. [Crossref] [PubMed]
- Winterbottom F, Seoane L, Sundell E, et al. Improving sepsis outcomes for acutely ill adults using interdisciplinary order sets. Clin Nurse Spec 2011;25:180-5. [Crossref] [PubMed]
- Ko HF, Tsui SS, Tse JW, et al. Improving the emergency department management of post-chemotherapy sepsis in haematological malignancy patients. Hong Kong Med J 2015;21:10-5. [PubMed]
- Zambon M, Ceola M, Almeida-de-Castro R, et al. Implementation of the Surviving Sepsis Campaign guidelines for severe sepsis and septic shock: we could go faster. J Crit Care 2008;23:455-60. [Crossref] [PubMed]
- Sebat F, Johnson D, Musthafa AA, et al. A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients. Chest 2005;127:1729-43. [Crossref] [PubMed]
- Nguyen SQ, Mwakalindile E, Booth JS, et al. Automated electronic medical record sepsis detection in the emergency department. PeerJ 2014;2:e343. [Crossref] [PubMed]
- Umscheid CA, Betesh J, VanZandbergen C, et al. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med 2015;10:26-31. [Crossref] [PubMed]
- Makam AN, Nguyen OK, Auerbach AD. Diagnostic accuracy and effectiveness of automated electronic sepsis alert systems: A systematic review. J Hosp Med 2015;10:396-402. [Crossref] [PubMed]
- Burrell AR, McLaws ML, Fullick M, et al. SEPSIS KILLS: early intervention saves lives. Med J Aust 2016;204:73.e1-7. [Crossref] [PubMed]
- Hooper MH, Weavind L, Wheeler AP, et al. Randomized trial of automated, electronic monitoring to facilitate early detection of sepsis in the intensive care unit*. Crit Care Med 2012;40:2096-101. [Crossref] [PubMed]
- Nelson JL, Smith BL, Jared JD, et al. Prospective trial of real-time electronic surveillance to expedite early care of severe sepsis. Ann Emerg Med 2011;57:500-4. [Crossref] [PubMed]
- Berger T, Birnbaum A, Bijur P, et al. A Computerized Alert Screening for Severe Sepsis in Emergency Department Patients Increases Lactate Testing but does not Improve Inpatient Mortality. Appl Clin Inform 2010;1:394-407. [Crossref] [PubMed]
- McRee L, Thanavaro JL, Moore K, et al. The impact of an electronic medical record surveillance program on outcomes for patients with sepsis. Heart Lung 2014;43:546-9. [Crossref] [PubMed]
- Semler MW, Weavind L, Hooper MH, et al. An Electronic Tool for the Evaluation and Treatment of Sepsis in the ICU: A Randomized Controlled Trial. Crit Care Med 2015;43:1595-602. [Crossref] [PubMed]
- Sawyer AM, Deal EN, Labelle AJ, et al. Implementation of a real-time computerized sepsis alert in nonintensive care unit patients. Crit Care Med 2011;39:469-73. [Crossref] [PubMed]
- Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015;372:1629-38. [Crossref] [PubMed]
- Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:775-87. [Crossref] [PubMed]
- Churpek MM, Snyder A, Han X, et al. qSOFA, SIRS, and Early Warning Scores for Detecting Clinical Deterioration in Infected Patients Outside the ICU. Am J Respir Crit Care Med 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Nguyen HB, Kuan WS, Batech M, et al. Outcome effectiveness of the severe sepsis resuscitation bundle with addition of lactate clearance as a bundle item: a multi-national evaluation. Crit Care 2011;15:R229. [Crossref] [PubMed]


