Fertility counseling of young breast cancer patients
Introduction
Approximately 3% of all tumours are diagnosed in patients younger than 40 years: the most common types of cancer in young women are breast carcinoma, tumours of the thyroid, melanoma, carcinoma of the cervix and carcinoma of the colon-rectum (1). Concerning breast cancer incidence, approximately 6% of women with breast carcinoma are diagnosed before the age of 40 (1); recent data showed that the incidence of breast cancer diagnosed in young women is increasing (2).
Although the majority of anticancer treatments (surgery, radiotherapy, chemotherapy, endocrine therapy and biologic therapy) have a substantial impact on gonadal function and may lead to loss of fertility (3), therapies performed to treat thyroid cancer and melanoma, generally, do not impair gonadal function. As reported by Stensheim et al. in a large population based study, the pregnancy rates in survivors of malignant melanoma or thyroid cancer are similar to that of general population (4). Conversely, a lower pregnancy rate occurred in survivors of breast cancer, cervical cancer and leukemia (4). The available evidence suggests that fertility preservation is becoming a primary issue for young cancer patients, and that infertility resulting from cancer treatment may be associated with psychosocial distress (3,5). The access to fertility counseling has a growing importance both for the improved prognosis of cancer patients and for the delaying of child-bearing that is a social problem in western nations (6). As recommended by the American Society of Clinical Oncology (ASCO), all oncologists should refer young cancer patients for fertility counseling: particularly, all patients should receive an assessment for and communication regarding risk of treatment-related infertility, and all patients at risk of infertility and interested in fertility preservation should be referred to a specialist with expertise in fertility preservations methods (3). Nevertheless, at least half of patients have no memory of a discussion about fertility at the time of their treatment disposition (7-11). The likelihood that oncologists discuss fertility preservation with newly diagnosed patients may be affected by patients’ characteristics such as prognosis, sex, age, marital status, sexual orientation and finances, but few data are available on this topic (7,12). Furthermore, some studies have suggested that oncologists may not know the clinical recommendations related to this issue or that their knowledge on the subject has little update (12,13); other studies report the negative effect of the lack of ad hoc multidisciplinary team (7,14). Fortunately, in recent years there is an improved understanding of the risks of infertility and of the available strategies to reduce its incidence, and a greater dissemination of information to both medical doctors and patients leading to more informed decision making and improved quality of care (15,16). As confirmed by a recent German study, the proportion of patients who do not remember any discussion about the issues related to fertility prior to treatment is gradually decreasing over time from 67% in the period 1980-1984 to 50% in the period 2000-2004 (17).
The main purpose of the present review is to encourage a reliable fertility counseling as a key moment in the decision-making process of young patients candidates for anticancer treatments. Data about pregnancy after breast cancer, the effect of anticancer treatments on gonadal function, the key points to keep in mind to perform a correct fertility counseling, and data about the available strategies for fertility preservations in breast cancer patients, will be reviewed.
Pregnancy after breast cancer
The proportion of patients with at least one full-term pregnancy after breast cancer diagnosis reported in the literature is very low: only 3% of women younger than 45 years at diagnosis (8% if considering only women aged less than 35 years) (18-21). This result is due to several factors including the damage derived from gonadotoxic therapy and the fear related to a negative impact of pregnancy on the evolution of breast cancer. There are two main concerns for young cancer patients to experience pregnancy after cancer diagnosis and treatment: the occurrence of congenital abnormalities and the potential obstetric and birth complications due to previous cancer treatments, and the possibility that pregnancy might have negative consequences on the prognosis of the patient herself.
Regarding the first point, data from four studies are available (22-25) (Table 1). The reported rate of congenital abnormalities of infants born to women with history of breast cancer ranges from 0% (23) to 7.2% (24). Considering that the percentage of congenital abnormalities in general population is nearly 4%, the rate observed in women with history of breast cancer is similar to that of general population in all (22,23,25) but one (24) of available studies. In the study by Dalberg et al. the congenital abnormalities reported were: ten cardiac defects (including three children with patent ductus arteriosus and four with septal defects), three kidney/ureteragenesis defects, two undescended testes in full-term infants, two unspecified limb malformations, two ear malformations, two skin malformations, one chromosome anomaly (trisomy 21), one congenital hydrocephaly, and one orofacial cleft (24).
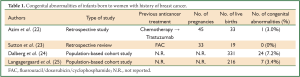
Full Table
Regarding to obstetric and birth complications a relatively higher abortion rate (20-44%) was reported in patients with history of breast cancer as compared to the untreated population (20,26-31). Such a higher abortion rate reflects the uncertainties and fear faced not only by patients but also by their threating physicians about the safety of pregnancy after the diagnosis and treatment of breast cancer (Table 2). Indeed, two recent cohort studies in a large population of women previously treated for breast cancer are reassuring (24,25), although the study by Dalberg et al. reported a higher incidence of birth complications, such as caesarean section, preterm birth, babies with low birth weight, in women previously treated for breast cancer as compared to controls (24). Therefore, a close monitoring of pregnancy in women previously treated for cancer is recommended.
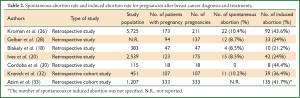
Full Table
With regard to the concern about the potential negative impact of pregnancy on patients’ prognosis, in the past, on the basis of purely theoretical assumptions, pregnancy after breast cancer was contraindicated. The available clinical data do not confirm such hypothesis: so far, it is well established that women who became pregnant after breast cancer do not have a worse prognosis (18-20,26,28,31-38). A meta-analysis of 14 retrospective control-matched studies that assessed the impact of pregnancies on overall survival (OS) of women with history of breast cancer, showed that women who got pregnant following breast cancer diagnosis had a 41% reduced risk of death compared to women who did not get pregnant [pooled relative risk (PRR): 0.59; confidence interval (CI): 0.50-0.70] (39). Even after correcting data for the so called “healthy mother effect”, in the subgroup analysis where the outcome of women with history of breast cancer who became pregnant was compared to breast cancer patients who did not get pregnant and were known to be free of relapse, there was no significant differences in survival between groups (PRR: 0.85; 95% CI: 0.53-1.35) (39).
To better clarify the impact of pregnancy on disease-free survival (DFS) in women with history of breast cancer according to estrogen receptor status, Azim et al. performed a multicenter retrospective cohort study (33). Patients who became pregnant any time after breast cancer were matched to patients with breast cancer with similar estrogen receptor, nodal status, adjuvant therapy, age and year at diagnosis: the primary objective was DFS in patients with estrogen receptor-positive breast cancer. No difference in DFS was observed between pregnant and non-pregnant patients in the estrogen receptor positive group [hazard ratio (HR): 0.91; 95% CI: 0.67-1.24] or the estrogen receptor negative cohort (HR: 0.75; 95% CI: 0.51-1.08). However the pregnant group had better overall survival (HR: 0.72; 95% CI: 0.54-0.97) with no interaction according to estrogen receptor status (33). So far, the historical contraindication to pregnancy in patients with previous history of breast cancer should be considered permanently dropped out, even if it is not clear yet the ideal interval to wait between the end of anticancer treatments and the conception. There are no biological rationale or supporting evidences to define a “gold standard time” for women to become subsequently pregnant (40). However, experts recommend avoiding early pregnancy within 2 years from diagnosis in case of high risk of early relapse (41). Timing could be “personalized” taking into accounts patient age, risk of relapse, previous treatments and need for adjuvant hormonal therapy (18,19,42). On this issue, a project carried on by the Breast International Group and North American Breast Cancer Group (BIG-NABCG) is going to start: it is a prospective study directed to young women with endocrine sensitive early breast cancer who desire to become pregnant and who are disease free after 2 years of adjuvant endocrine therapy (38). The major aims of the projects are to assess patients and offspring outcomes, focusing on pregnancy (abortion, miscarriage, ectopic stillbirth, live birth rates), birth (preterm birth, low birth weight, birth defects rates) and breast cancer outcomes (DFS, OS). The trial is divided in two phases: (I) the observational phase investigates the feasibility and impact of a temporary treatment interruption to allow conception; (II) the subsequent experimental phase will investigate the optimal duration of subsequent endocrine treatment after delivery (38).
Reassurance on the safety of pregnancy in patients who experienced breast cancer is increasing the number of couples who have access to the Centers of Reproductive Medicine because of infertility after cancer treatments. Even though assisted reproduction may be an option for those couples with other infertility factors (such as tubal factor, endometriosis, male factor, etc.) when infertility is due to reduced ovarian function because of gonadotoxic therapies, reduced success are obtained compared with non-cancer patients (43).
Effect of anticancer treatments on gonadal function
Infertility is defined as the inability to conceive after 1 year of intercourse without contraception.
Anticancer treatment may have a negative impact on gonadal function and may lead to loss of fertility and early menopause. Acute amenorrhea occurring during treatment, may be affected permanently or temporary and results from loss of the growing follicle population. The majority of patients younger than 40 years recover menses within 1 year from cessation of treatment; incidence of permanent amenorrhea after systemic treatment for breast cancer is estimated to be between 33% and 76% in women age 50 or younger (44). However since the primordial follicle pool is bound to be reduced also in women who resume menses, patients should be advised of a higher risk of infertility and premature menopause to let them make a well-timed family planning. It has been demonstrated that women who continue to menstruate after treatment with chemotherapy for breast cancer remain at an increased risk of entering menopause early and that a significative reduction of fertility potential anticipate menopause of about 5 years (45).
The effects of anticancer treatments on reproductive organs may be direct (e.g., pelvic surgery or irradiation, chemotherapy) or may derive by hormonal alteration (e.g., a cranial irradiation damaging the pituitary axis) (16). The rate of anticancer treatment-related infertility is variable and depends on several factors: class, dose, dose-intensity of the drug used, method of administration (oral versus intravenous), size and location of the radiation field, the radiation delivered dose and its fragmentation, age of the patient, disease, history of previous treatment for infertility, comorbidities (3).
Particularly, the incidence of anticancer-treatment-related ovarian failure in breast cancer patients depends mainly on the type of chemotherapy regimen administered, the use of tamoxifen and the age of patients at diagnosis. It rises with increasing age, in the range of 22-61% and 61-97% in women aged 40 years respectively (46). Among chemotherapy agents, the greatest risk is associated with alkylating agents (particularly cyclophosphamide) (47-49); also carboplatin and cisplatin can have a negative effect. A low risk of treatment-related ovarian failure is associated with methotrexate (M) and fluorouracil (F) (3). Few data are available for newer agents such as taxanes. Fornier et al. reported a case series of 230 women younger than 40 years treated with the addition of taxanes to anthracycline-containing chemotherapy for breast cancer showing a similar rate of amenorrhea for this women compared to historical controls (50). However, available date about the risk of amenorrhea with taxanes are still not conclusive (51).
Focusing on clinical studies in breast cancer patients, the incidence of chemotherapy-induced amenorrhea by regimen ranged from 9% to 75% (Table 3) (50,52-56). Ganz and colleagues provided results of the menstrual history (MH) and quality-of-life (QoL) outcomes in breast cancer patients treated with adjuvant therapy within the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-30 trial (56). The NSABP B-30 trial was a three-arm multicenter study carried on in 5,300 women with early-stage, node-positive breast cancer: it demonstrated that adjuvant therapy with sequential doxorubicin (A) and cyclophosphamide (C) followed by docetaxel (T; AC→T), compared with four cycles of AT or TAC, improved DFS and OS (57). MH and QoL were secondary outcomes of the trial and were assessed with standardized questionnaires at baseline and at follow-up visits every 6 months (56). Pre-specified analyses evaluated rates of amenorrhea by treatment arm, the relationship between amenorrhea and QoL, and QoL by treatment arm. Prolonged amenorrhea was defines as having at least 6 months without a menstrual cycle. The rates of prolonged amenorrhea at 12 months after the start of therapy was significantly different between treatment arms: 69.8% for AC→T, 37.9% for AT, and 57.7% for TAC (P<0.001). The amenorrhea rates were higher with the addition of tamoxifen; the AT group without tamoxifen showed the lowest rate of amenorrhea, hovering around 20-30% across the 24-month period of observation. Approximately 61% of women under the age of 40 experienced at least 24 months of amenorrhea contrasting with nearly 100% among patients older than 40 years (56). This study highlighted that, among chemotherapy agents, alkylating agents are associated with a high gonadal toxicity. There are two major mechanisms associated with chemotherapy induced ovarian toxicity, the direct induction of follicle and oocyte apoptosis (58) and the vascular damage to the ovary (59). Compared to untreated women, patients receiving chemotherapy showed a significantly lower follicle counts (58). Such an effect was more pronounced in patients receiving alkylating agents than in patients who did not receive these agents (58). Moreover, chemotherapy regimens, regardless of whether they include an alkylating agent, showed to alter also ovarian stromal function: ovarian cortical pieces that were previously exposed to chemotherapy secreted significantly less estradiol compared with controls (58). Injury to blood vessels and focal damage to the ovarian cortex are considered other important mechanisms for chemotherapy induced ovarian toxicity (59). Ovarian tissue previously exposed to chemotherapy, showed to have severe narrowing and obliteration of the vascular lumen of cortical blood vessels, due to hyalinization of the vessel, intimal fibrosis and thickening of the muscular layer; furthermore, ovaries exposed to chemotherapy revealed several areas of subcapsular focal cortical fibrosis with preservation of the ovarian surface epithelium (59).
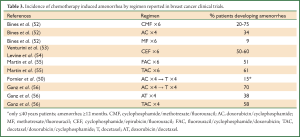
Full Table
Concerning adjuvant endocrine therapy, tamoxifen alone is associated with a low risk of premature menopause, which is strictly dependent on age: over the age of 45, the risk of infertility is 10% higher than in controls (60). The administration of tamoxifen sequentially to chemotherapy causes a statistically significant increase in the risk of infertility compared to chemotherapy alone (53,61). The analogues of luteinizing hormone (LHRHa) or gonadotropin-releasing hormone (GnRHa) lead to a temporary ovarian suppression; however, the reversibility of such effect is strongly influenced by patients’ age: the resumption of menstrual cycles is expected in 90% of patients under the age of 40, and in 70% of women older than 40 years (19,60).
Age, the specific chemotherapy regimen administered and marginal tamoxifen use have a very important impact on ovarian function in young cancer patients. This finding has been confirmed by Petrek and colleagues in a prospective observational study that assessed ovarian function after breast cancer treatment in 595 premenopausal patients (62). Ovarian function was assessed using the surrogate of monthly bleeding; median follow-up was 45 months. Patients of all ages experienced disruptions in their menstrual activity: however, the majority of women aged 40 years or older had no menstrual bleeding at the end of chemotherapy and no recovery of bleeding in the follow up years compared with younger women. Patients younger than 35 years had rapid menstrual cycling recovery with the proportion with bleeding rising to approximately 85% at 6 months following the end of chemotherapy, and remaining relatively constant; the recovery was less pronounced for patients between the ages of 35 and 40. Concerning the chemotherapy regimen administered, treatment with AC alone resulted in an important decrease in the proportion of patients with periods; paclitaxel or T added to AC led to a small further decline in the number of patients with bleeding, while CMF resulted in a greater proportion of patients with monthly bleeding in the initial months but with a progressive decrease in the follow-up years. Finally, the addition of tamoxifen resulted in a decrease in the proportion of patients with monthly bleeding by 1 year following chemotherapy, but this effect became non significant by 3 years. In conclusion, significantly different proportions of women had monthly bleeding depending on their age (P<0.001), chemotherapy regimen (P<0.001), and time since chemotherapy. Using monthly bleeding as surrogate to assess ovarian function, authors showed the important impact of age, chemotherapy regimen and tamoxifen use, on gonadal function of young breast cancer patients (62).
Key issues during fertility counseling
The possible impact of anticancer treatment on fertility and menstrual function should be addressed in all breast cancer patients in reproductive age (3). The choice of the best time to discuss issues related to chemotherapy induced infertility risks is complex. In general, an early discussion facilitates the planning of a fertility preservation technique. Particularly, oocyte or embryo cryopreservation needs a couple of weeks from the beginning of a menstrual cycle to be accomplished with the consequence that therapy initiation can be delayed for more than a month. Moreover a young patient who finds out to have a cancer and at the same time is plugged into the decision of whether undergoing a fertility preservation technique, needs some time to make her decision. On the other hand, a premature referral to reproductive counseling may overestimate the need of fertility preservation strategies in the cases that will not require chemotherapy, increasing unnecessarily the psychological burden for these patients. Physician first addresses fertility issues in cancer patients must be aware of the above mentioned pitfalls related to various counseling timing (last menstrual period and expected final diagnosis).
It is responsibility of the radiologist, surgeon and, mainly, of the oncologist to make patients aware of the impact of cancer treatment on fertility and to evaluate if they wish a thorough reproductive counseling (Figure 1). Oncologists with enough experience and knowledge in this field, may carry on a complete reproductive counseling themselves and refer to Reproductive Units only those patients who choose to undergo cryopreservation fertility techniques. Oncologists need to have a cooperation with one or more Reproductive Units to give their patients the opportunity to undergo a well-timed and complete counseling (12,13). Therefore, a well-organized linkage between oncology and Reproductive Units is the first step to be accomplished to face the management of fertility issues in cancer patients (Figure 1).
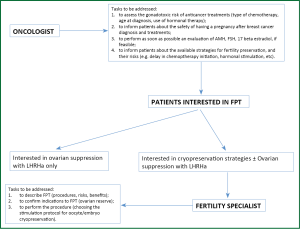
According to results of a recent survey on post-treatment QoL that included 1,041 women aged 18-40 years who were counseled either by the oncology team (61%) or by fertility specialists (5%), specialized counseling about reproductive loss and pursuing fertility preservation is associated with less regret and greater QoL for survivors (63). In this study 36 (4%) patients took action to preserve fertility (63).
Fertility counseling should be patient-tailored, since both the impact of chemotherapy on reproductive potential and the success of fertility preservation techniques are strongly linked to patient’s age and ovarian reserve. Ovarian reserve is a widely used term to indicate the ovary reproductive potential due to the number and the quality of its oocytes asset (64). Many factors, in addition to age, may affect ovarian reserve and consequently the expected damage induced by chemotherapy, and the success of fertility preservation techniques. Some of these factors, such as multiple ovarian surgery, heavy smoking, progressively shorter cycle duration, family history of premature menopause, are suggested by a proper clinical history collection and should be searched for (65). Ovarian reserve is assessed by hormonal assays and evaluation of antral follicular count (AFC) with transvaginal ultrasound (66). Among hormonal markers, anti-mullerian hormone (AMH) has been proven the more accurate in predicting ovarian response to stimulation both in IVF than in fertility preservation cycles (67,68). It is a dimeric glycoprotein produced by granulosa cells, from pre-antral and antral follicles and reflects the ovarian follicular pool. AMH concentration measurements are useful in the evaluation of chemotherapy induced ovarian damage and may become a tool for the comparison of ovarian toxicity of different chemotherapy regimens (69-72). Since AMH concentrations are stable throughout the menstrual cycle, differently from other hormonal markers such as basal follicle-stimulating hormone (FSH) and 17 beta estradiol which must be dosed early in the follicular phase (day 2-4), AMH evaluation should be done as soon as possible to make results available at the time of consultation. Patients’ age and ovarian reserve markers measurement are essential to estimate expected damage of anticancer therapies on ovarian function and to decide about fertility preservation techniques (73).
Adequate efficiency of both oocyte/embryo and ovarian tissue cryopreservation can be expected in patients below 38 years of age and with an age-appropriate ovarian reserve. In patients aged between 38 and 40, fertility preservation techniques may be efficacious only in cases with a good ovarian reserve. It has been reported a low response to stimulation with letrozole and gonadotropins for oocytes recovery in breast cancer patients when the AMH level is ≤1.2 ng/mL (68).
Patients should be informed that chemotherapy to treat breast cancer implies a risk of ovarian function compromise that include acute ovarian failure, infertility and early menopause, which probably are three different signs of the same mechanism. It is essential that patients understand that their reproductive potential may be impaired also in the presence of regular menses (74).
Fertility counseling should include a detailed description of all the available techniques to preserve fertility which are appropriate for that particular patient including procedures, timing, possible complications, expected results. It is mandatory to make clear to the patient what is well-known and what is still experimental about these techniques. In some cases, more than one technique can be applied at the same patient or, when chemotherapy can be postponed, more cycles of ovarian stimulation can be performed to storage a larger number of oocytes or embryos rising the chances of future pregnancies. There are some circumstances which may increase complications or contraindicate a technique such as thromboembolic risk, severe abdominal adhesions which must be taken into consideration during fertility counseling.
The percentage of patients who choose to undergo oocyte/embryo or ovarian tissue cryopreservation after fertility counseling reported in the literature varies from 4% to over 50% (63,75). In our experience approximately 22% of breast cancer patients accepted to undergo fertility counseling performed by the reproductive physician and 8% underwent surgical fertility preservation techniques (oocytes cryopreservation or ovarian tissue cryopreservation) (76). A better understanding of factors that influence patients’ choice will help physicians to improve the quality of fertility counseling.
Strategies for fertility preservation
The choice between the available fertility preservation strategies for young women candidates for cancer treatments depends on several factors: patient’s age and ovarian reserve, type of cancer treatment planned, whether she has a partner, the time available, and the possibility that cancer has metastasized to her ovaries (77).
So far, the main available fertility preservation techniques, standard and experimental, for young breast cancer patients are: temporary ovarian suppression, embryo cryopreservation, cryopreservation of oocytes and cryopreservation of ovarian tissue (Table 4). Among the cryopreservation techniques, to date, cryopreservation of embryos and of mature oocytes are the only strategies that have shown reliable results, while cryopreservation of ovarian tissue or cryopreservation of immature oocyte or of oocytes matured in vitro are still in the early experimental phase.
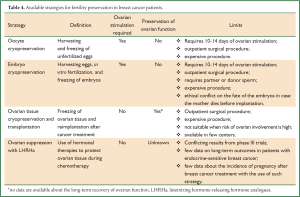
Full Table
Ovarian suppression with LHRHa
The rationale for the use of LHRHa to reduce the gonadal toxicity of chemotherapy is the observation that cytotoxic drugs mostly affect tissues with a rapid cellular turnover; then, a state of induced gonadal inhibition during exposure to chemotherapy may protect the ovaries (78). Because chronic administration of LHRHa decreases FSH secretion and suppresses gonadal function, it has been hypothesized that it may reduce chemotherapy toxicity on the gonads (79). Four phase III studies have recently been published in breast cancer patients candidates for chemotherapy to investigate the efficacy of such strategy to preserve ovarian function (80-83). In these studies, breast cancer patients were randomly assigned to receive adjuvant or neoadjuvant chemotherapy in combination with LHRHa or chemotherapy alone. These studies reported conflicting results. Major limits of these studies are: heterogeneous target population and differences in patients’ age at treatment, chemotherapy regimens used, selection of patients, duration of follow-up, and end points utilized to assess treatment efficacy. A recent meta-analysis to evaluate the role of LHRHa in the prevention of chemotherapy-induced premature ovarian failure (POF) has been presented: a total of seven randomized clinical trials involving 745 premenopausal patients randomly assigned to receive chemotherapy or chemotherapy plus LHRHa were included in the analysis; 5 trials were carried out in breast cancer patients and two trials in lymphoma patients (84). The pooled odds ratio estimate for chemotherapy induced POF was 0.46 (95% CI: 0.3-0.72) showing an important benefit of this strategy in reducing the gonadal toxicity of cytotoxic therapy in premenopausal cancer patients (84). Recently, a meta-analysis designed to assess the efficacy of LHRHa administration to prevent chemotherapy induced ovarian toxicity specifically in premenopausal breast cancer women has been published (85). Five randomized clinical trials (total number of patients: 528) were included in the analysis: significantly fewer women treated with LHRHa during chemotherapy experienced post-treatment POF (RR: 0.40; 95% CI: 0.21-0.75). However, both treatment groups had similar rates of resumed menses (RR: 1.31; 95% CI: 0.93-1.85) and spontaneous pregnancy (RR: 0.96; 95% CI: 0.20-4.56) (85).
This strategy, in contrast to embryo and oocyte cryopreservation, can preserve the overall ovarian function and not only fertility; furthermore, this technique can be performed in combination with cryopreservation strategies, thus increasing the chance of fertility recovery after cancer treatments.
This strategy has two major limits: few data are available on the long term efficacy and safety of the technique, and there is no reimbursement of these treatment by the National Health System of most countries even if the cost of the treatment is lower (about 1,000 euros for 6 months of treatment) than the cost of cryopreservation strategies.
Embryo or oocyte cryopreservation
One of these two strategies is recommended as fertility preservation option in breast cancer patients (86). Cryopreservation of embryos has been the only established procedure for fertility preservation for many years; since January 2013, cryopreservation of oocytes is no longer considered experimental (87). Cryopreservation of oocytes can be applied also in patients without a male partner and in countries where embryo cryopreservation is prohibited. Both techniques may be offered when it is medically reasonable to delay chemotherapy by 2 to 6 weeks because they require a phase of ovarian stimulation lasting about 9-15 days which is usually started at the onset of menses (3). Moreover, since efficacy of oocyte and embryo cryopreservation depends on the number of recovered oocytes, these procedures may be proposed only to patients below the age of 38-40 years and with the possibility to recover a sufficient number of oocytes (approximately 8-15).
To overcome the need to wait the onset of menses and allow more patients the chance of embryo/oocyte cryopreservation without delaying initiation of chemotherapy, there are some attempts with the initiation of ovarian stimulation in the luteal or late follicular phases. Preliminary experiences with these “emergency protocols” showed promising results in terms of oocyte recovery (88-90).
There are still some concerns about the impact of the ovarian stimulation required for oocyte and embryo cryopreservation, on hormone responsive tumors. To reduce the potential risk of short-term exposure to high estrogen levels alternative approaches for ovarian stimulation with letrozole or tamoxifen has been developed (91-93). The largest experience with the use of cryopreservation strategies in breast cancer patients is reported by Azim and colleagues (94). Authors prospectively evaluated for fertility preservation 215 breast cancer patients before adjuvant chemotherapy: a total of 79 women underwent embryo or oocyte cryopreservation, and the remained 136 patients did not undergo any fertility-preserving procedures and served as controls (94). At a median follow up of 23.4 months after chemotherapy the HR for recurrence after in vitro fertilization (IVF) was 0.56 (95% CI: 0.17-1.9) and the survival of patients that underwent cryopreservation strategies was not compromised compared with controls (P=0.36). As reported in the conclusion of the paper, “further research, including longer-term follow-up is needed to confirm these findings” (94).
There are few data on pregnancies obtained with oocyte and embryo cryopreserved in cancer patients: therefore, to estimate pregnancy rate potential of these fertility preservation techniques it is necessary to consider data derived from the age-matched infertile population (95). Moreover, during fertility counseling, clinic-specific success rate should be considered, since results varies among different laboratories.
Among emerging strategies to be considered still experimental, cryopreservation of immature oocyte or of oocytes matured in vitro should be mentioned. Through this techniques, oocytes’ collection can be obtained without hormonal stimulation or with a short stimulation lasting 3-5 days; immature oocytes can be cryopreserved after maturation in vitro or cryopreserved at the immature stage and then matured in vitro after thaw before insemination. So far, the results obtained with these strategies are lower than those obtained with oocytes matured in vivo (96,97).
Ovarian tissue cryopreservation
This is a promising technique but should be considered still experimental (3). Major advantages are that it does not require neither a sperm donor nor hormonal stimulation, and that it offers the opportunity to preserve both fertility and the overall ovarian function. This strategy can be performed at any time of the menstrual cycle, thus avoiding the delay in chemotherapy initiation; however, it requires a laparoscopic surgery for the removal of fragments of ovarian cortex (98,99). This strategy can be offered to patients younger than 38 years with adequate ovarian reserve: the success rate of the technique in older women is uncertain due to the reduced number of primordial follicles at that age (100). Ovarian tissue is removed through a laparoscopic procedure requiring general anesthesia, and then frozen (3). A large biopsy is needed because many follicles are lost during freezing/thawing/transplantation procedures (101). The ovarian tissue, once the patient has completed cancer treatment, can be transplanted orthotopically to the pelvis (102-106) or heterotopically to subcutaneous areas (for example forearm, lower abdomen) (107,108). To date, more than 25 pregnancies have been reported, all of them after orthotopic grafting, either spontaneously or with assisted reproductive technique. However, it is not possible to express the success rate for autotransplantation of cryopreserved ovarian tissue, as it is not well known how many attempts have been made of reimplantation of thawed frozen ovarian tissue in women.
In a single center experience, four of the seven patients who underwent ovarian tissue transplantation, conceived with assisted reproduction techniques (57%) (109). The percentage of ovarian function recovery is high (90-100%) even if its duration is still limited (up to a few years) (110).
One important concern about the application of this technique is the potential reintroduction of cancer cells (111-113). In a recent large study aiming to assess the incidence of malignant cells in ovarian tissue before cryopreservation, 1.3% (5/391) of ovarian tissue samples were found positive for malignant cells at light microscopy evaluation (114). All positive samples belonged to patients with haematologic disease while so far, no malignant cells have been found in ovarian tissue from breast cancer patients by immunohistochemistry (115,116). However, it is essential to provide an adequate preoperative screening to rule out a possible cancer involvement of the ovary and to perform an accurate histological examination of the ovarian tissue removed before replanting it (115-119).
So far, ovarian tissue cryopreservation has to be considered still experimental and should be performed only in centers with the necessary expertise under approved clinical protocols; furthermore, particular attention should be paid to the follow-up of these patients for recurrent cancer (3).
Conclusions
Loss of reproductive potential as a consequence of anticancer treatment negatively impacts QoL in young survivors (120,121). As showed in recent studies, the potential iatrogenic loss of fertility, which also means loss of a potential child, has a profound impact on young women and in some ways may be more stressful than the cancer diagnosis itself (122,123). So far, all oncologists should refer young cancer patients for fertility counseling: receiving counseling about reproductive loss before anticancer therapies significantly improved QoL after cancer treatment for reproductive-age women (63). Particularly, all patients with newly diagnosed cancer should receive an assessment for and communication regarding risk of treatment-related infertility, and all patients interested in fertility preservation should be referred to a specialist with expertise in fertility preservations methods (3). Since the historical contraindication to pregnancy in patients with previous history of breast cancer should be considered permanently dropped out, the same recommendation should be applied to breast cancer patients. As showed by Rippy et al., an active approach to counseling makes a huge psychological difference (124). Authors assessed in women under the age of 45 at the time of diagnosis of breast cancer, how many of them wanted and tried to become pregnant after breast cancer treatment, the effect of pre-treatment counseling and their prognosis. They showed a higher rate of pregnancy than expected, possibly due to newer treatments including fertility preservation and also possibly due to the active counseling program in the unit. Authors concluded that “the positive attitude of the breast team towards pregnancy may also help reduce the fear of pregnancy after breast cancer and consequently also reduce the elective abortion rate” (124).
Oncologists should feel empowered to discuss the possible fertility loss due to anticancer treatments and the available strategies to reduce such effect. Patients should have active counseling about fertility when planning treatment, and fertility preservation can then be incorporated into a treatment plan. An informed choice about whether to access any available fertility preservation strategy can only be made after a proper discussion of their risks, success rates and costs. On the other hand, being some fertility preservation strategies still experimental and difficult to access in some centers, there is an imperative for oncologists and gynecologists to conduct more research efforts in this important field (125). Major attention should be performed to obtain data on the long term follow up of breast cancer patients that underwent one or more fertility preservation strategies at the time of cancer diagnosis and treatment. More research are needed to improve the efficacy and safety of the available strategies, and an effective collaboration between oncologists and gynecologists should be implemented to improve patients access to reproductive technologies.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Surveillance, Epidemiology and End Results (SEER) Web site. Available online: http://www.seer.cancer.gov. April 2010, based on the November 2009 submission.
- Merlo DF, Ceppi M, Filiberti R, et al. Breast cancer incidence trends in European women aged 20-39 years at diagnosis. Breast Cancer Res Treat 2012;134:363-70. [PubMed]
- Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006;24:2917-31. [PubMed]
- Stensheim H, Cvancarova M, Møller B, et al. Pregnancy after adolescent and adult cancer: a population-based matched cohort study. Int J Cancer 2011;129:1225-36. [PubMed]
- Reh AE, Lu L, Weinerman R, et al. Treatment outcomes and quality-of-life assessment in a university-based fertility preservation program: results of a registry of female cancer patients at 2 years. J Assist Reprod Genet 2011;28:635-41. [PubMed]
- Johnson JA, Tough S, Society of Obstetricians and Gynaecologists of Canada. Delayed child-bearing. J Obstet Gynaecol Can 2012;34:80-93. [PubMed]
- Schover LR, Brey K, Lichtin A, et al. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol 2002;20:1880-9. [PubMed]
- Schover LR, Rybicki LA, Martin BA, et al. Having children after cancer. A pilot survey of survivors’ attitudes and experiences. Cancer 1999;86:697-709. [PubMed]
- Zebrack BJ, Casillas J, Nohr L, et al. Fertility issues for young adult survivors of childhood cancer. Psychooncology 2004;13:689-99. [PubMed]
- Duffy CM, Allen SM, Clark MA. Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol 2005;23:766-73. [PubMed]
- Snyder KA, Pearse W. Discussing fertility preservation options with patients with cancer. JAMA 2011;306:202-3. [PubMed]
- Quinn GP, Vadaparampil ST, Gwede CK, et al. Discussion of fertility preservation with newly diagnosed patients: oncologists’ views. J Cancer Surviv 2007;1:146-55. [PubMed]
- Köhler TS, Kondapalli LA, Shah A, et al. Results from the survey for preservation of adolescent reproduction (SPARE) study: gender disparity in delivery of fertility preservation message to adolescents with cancer. J Assist Reprod Genet 2011;28:269-77. [PubMed]
- Quinn GP, Vadaparampil ST, Lee JH, et al. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol 2009;27:5952-7. [PubMed]
- Partridge AH, Ruddy KJ, Kennedy J, et al. Model program to improve care for a unique cancer population: young women with breast cancer. J Oncol Pract 2012;8:e105-10. [PubMed]
- Ruddy KJ, Partridge AH. Fertility (male and female) and menopause. J Clin Oncol 2012;30:3705-11. [PubMed]
- Hohmann C, Borgmann-Staudt A, Rendtorff R, et al. Patient counselling on the risk of infertility and its impact on childhood cancer survivors: results from a national survey. J Psychosoc Oncol 2011;29:274-85. [PubMed]
- Blakely LJ, Buzdar AU, Lozada JA, et al. Effects of pregnancy after treatment for breast carcinoma on survival and risk of recurrence. Cancer 2004;100:465-9. [PubMed]
- Del Mastro L, Catzeddu T, Venturini M. Infertility and pregnancy after breast cancer: current knowledge and future perspectives. Cancer Treat Rev 2006;32:417-22. [PubMed]
- Ives A, Saunders C, Bulsara M, et al. Pregnancy after breast cancer: population based study. BMJ 2007;334:194. [PubMed]
- Mueller BA, Simon MS, Deapen D, et al. Childbearing and survival after breast carcinoma in young women. Cancer 2003;98:1131-40. [PubMed]
- Azim HA Jr, Metzger-Filho O, de Azambuja E, et al. Pregnancy occurring during or following adjuvant trastuzumab in patients enrolled in the HERA trial (BIG 01-01). Breast Cancer Res Treat 2012;133:387-91. [PubMed]
- Sutton R, Buzdar AU, Hortobagyi GN. Pregnancy and offspring after adjuvant chemotherapy in breast cancer patients. Cancer 1990;65:847-50. [PubMed]
- Dalberg K, Eriksson J, Holmberg L. Birth outcome in women with previously treated breast cancer--a population-based cohort study from Sweden. PLoS Med 2006;3:e336. [PubMed]
- Langagergaard V, Gislum M, Skriver MV, et al. Birth outcome in women with breast cancer. Br J Cancer 2006;94:142-6. [PubMed]
- Kroman N, Jensen MB, Melbye M, et al. Should women be advised against pregnancy after breast-cancer treatment? Lancet 1997;350:319-22. [PubMed]
- Velentgas P, Daling JR, Malone KE, et al. Pregnancy after breast carcinoma: outcomes and influence on mortality. Cancer 1999;85:2424-32. [PubMed]
- Gelber S, Coates AS, Goldhirsch A, et al. Effect of pregnancy on overall survival after the diagnosis of early-stage breast cancer. J Clin Oncol 2001;19:1671-5. [PubMed]
- Lawrenz B, Henes M, Neunhoeffer E, et al. Pregnancy after successful cancer treatment: what needs to be considered? Onkologie 2012;35:128-32. [PubMed]
- Córdoba O, Bellet M, Vidal X, et al. Pregnancy after treatment of breast cancer in young women does not adversely affect the prognosis. Breast 2012;21:272-5. [PubMed]
- von Schoultz E, Johansson H, Wilking N, et al. Influence of prior and subsequent pregnancy on breast cancer prognosis. J Clin Oncol 1995;13:430-4. [PubMed]
- Kranick JA, Schaefer C, Rowell S, et al. Is pregnancy after breast cancer safe? Breast J 2010;16:404-11. [PubMed]
- Azim HA Jr, Kroman N, Paesmans M, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol 2013;31:73-9. [PubMed]
- Kroman N, Jensen MB, Wohlfahrt J, et al. Pregnancy after treatment of breast cancer--a population-based study on behalf of Danish Breast Cancer Cooperative Group. Acta Oncol 2008;47:545-9. [PubMed]
- Sankila R, Heinävaara S, Hakulinen T. Survival of breast cancer patients after subsequent term pregnancy: “healthy mother effect Am J Obstet Gynecol 1994;170:818-23. [PubMed]
- Malamos NA, Stathopoulos GP, Keramopoulos A, et al. Pregnancy and offspring after the appearance of breast cancer. Oncology 1996;53:471-5. [PubMed]
- Ariel IM, Kempner R. The prognosis of patients who become pregnant after mastectomy for breast cancer. Int Surg 1989;74:185-7. [PubMed]
- Pagani O, Partridge A, Korde L, et al. Pregnancy after breast cancer: if you wish, ma’am. Breast Cancer Res Treat 2011;129:309-17. [PubMed]
- Azim HA Jr, Santoro L, Pavlidis N, et al. Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer 2011;47:74-83. [PubMed]
- Litton JK. Breast cancer and fertility. Curr Treat Options Oncol 2012;13:137-45. [PubMed]
- Cardoso F, Loibl S, Pagani O, et al. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer 2012;48:3355-77. [PubMed]
- Royal College of obstetricians and gynaecologists. Pregnancy and breast cancer. Guideline No. 12; Jan 2004. Available online: http://www.rcog.org.uk/index.sap?PageID=529
- Barton SE, Missmer SA, Berry KF, et al. Female cancer survivors are low responders and have reduced success compared with other patients undergoing assisted reproductive technologies. Fertil Steril 2012;97:381-6. [PubMed]
- Hulvat MC, Jeruss JS. Maintaining fertility in young women with breast cancer. Curr Treat Options Oncol 2009;10:308-17. [PubMed]
- Partridge A, Gelber S, Gelber RD, et al. Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer 2007;43:1646-53. [PubMed]
- Del Mastro L, Venturini M, Sertoli MR, et al. Amenorrhea induced by adjuvant chemotherapy in early breast cancer patients: prognostic role and clinical implications. Breast Cancer Res Treat 1997;43:183-90. [PubMed]
- Howell S, Shalet S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol Metab Clin North Am 1998;27:927-43. [PubMed]
- Fisher B, Dignam J, Mamounas EP, et al. Sequential methotrexate and fluorouracil for the treatment of node-negative breast cancer patients with estrogen receptor-negative tumors: eight-year results from National Surgical Adjuvant Breast and Bowel Project (NSABP) B-13 and first report of findings from NSABP B-19 comparing methotrexate and fluorouracil with conventional cyclophosphamide, methotrexate, and fluorouracil. J Clin Oncol 1996;14:1982-92. [PubMed]
- Longhi A, Macchiagodena M, Vitali G, et al. Fertility in male patients treated with neoadjuvant chemotherapy for osteosarcoma. J Pediatr Hematol Oncol 2003;25:292-6. [PubMed]
- Fornier MN, Modi S, Panageas KS, et al. Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer 2005;104:1575-9. [PubMed]
- Okanami Y, Ito Y, Watanabe C, et al. Incidence of chemotherapy-induced amenorrhea in premenopausal patients with breast cancer following adjuvant anthracycline and taxane. Breast Cancer 2011;18:182-8. [PubMed]
- Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol 1996;14:1718-29. [PubMed]
- Venturini M, Del Mastro L, Aitini E, et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: results from a randomized trial. J Natl Cancer Inst 2005;97:1724-33. [PubMed]
- Levine MN, Bramwell VH, Pritchard KI, et al. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1998;16:2651-8. [PubMed]
- Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 2005;352:2302-13. [PubMed]
- Ganz PA, Land SR, Geyer CE Jr, et al. Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol 2011;29:1110-6. [PubMed]
- Swain SM, Jeong JH, Geyer CE Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med 2010;362:2053-65. [PubMed]
- Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007;110:2222-9. [PubMed]
- Meirow D, Dor J, Kaufman B, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod 2007;22:1626-33. [PubMed]
- Goodwin PJ, Ennis M, Pritchard KI, et al. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 1999;17:2365-70. [PubMed]
- Lee S, Kil WJ, Chun M, et al. Chemotherapy-related amenorrhea in premenopausal women with breast cancer. Menopause 2009;16:98-103. [PubMed]
- Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol 2006;24:1045-51. [PubMed]
- Letourneau JM, Ebbel EE, Katz PP, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer 2012;118:1710-7. [PubMed]
- Gleicher N, Weghofer A, Barad DH. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol 2011;9:23. [PubMed]
- Abusief ME, Missmer SA, Ginsburg ES, et al. Relationship between reproductive history, anthropometrics, lifestyle factors, and the likelihood of persistent chemotherapy-related amenorrhea in women with premenopausal breast cancer. Fertil Steril 2012;97:154-9. [PubMed]
- Lutchman Singh K, Muttukrishna S, Stein RC, et al. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer 2007;96:1808-16. [PubMed]
- La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 2010;16:113-30. [PubMed]
- Lee S, Ozkavukcu S, Heytens E, et al. Anti-Mullerian hormone and antral follicle count as predictors for embryo/oocyte cryopreservation cycle outcomes in breast cancer patients stimulated with letrozole and follicle stimulating hormone. J Assist Reprod Genet 2011;28:651-6. [PubMed]
- Anderson RA, Themmen AP, Al-Qahtani A, et al. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod 2006;21:2583-92. [PubMed]
- Lie Fong S, Lugtenburg PJ, Schipper I, et al. Anti-müllerian hormone as a marker of ovarian function in women after chemotherapy and radiotherapy for haematological malignancies. Hum Reprod 2008;23:674-8. [PubMed]
- Anderson RA, Cameron DA. Pretreatment serum anti-müllerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab 2011;96:1336-43. [PubMed]
- Morgan S, Anderson RA, Gourley C, et al. How do chemotherapeutic agents damage the ovary? Hum Reprod Update 2012;18:525-35. [PubMed]
- Rosendahl M, Andersen CY, la Cour Freiesleben N, et al. Dynamics and mechanisms of chemotherapy-induced ovarian follicular depletion in women of fertile age. Fertil Steril 2010;94:156-66. [PubMed]
- Letourneau JM, Ebbel EE, Katz PP, et al. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer 2012;118:1933-9. [PubMed]
- Lawrenz B, Jauckus J, Kupka MS, et al. Fertility preservation in >1,000 patients: patient’s characteristics, spectrum, efficacy and risks of applied preservation techniques. Arch Gynecol Obstet 2011;283:651-6. [PubMed]
- Lambertini M, Anserini P, Fontana V, et al. Prospective observational study on fertility preservation in young early breast cancer patients: the PREFER (PREgnancy and FERtility) trial. 2013 ASCO Annual Meeting (abstract: e17548).
- Roberts JE, Oktay K. Fertility preservation: a comprehensive approach to the young woman with cancer. J Natl Cancer Inst Monogr 2005;57-9. [PubMed]
- Rivkees SA, Crawford JD. The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA 1988;259:2123-5. [PubMed]
- Del Mastro L, Giraudi S, Levaggi A, et al. Medical approaches to preservation of fertility in female cancer patients. Expert Opin Pharmacother 2011;12:387-96. [PubMed]
- Badawy A, Elnashar A, El-Ashry M, et al. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril 2009;91:694-7. [PubMed]
- Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA 2011;306:269-76. [PubMed]
- Gerber B, von Minckwitz G, Stehle H, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol 2011;29:2334-41. [PubMed]
- Munster PN, Moore AP, Ismail-Khan R, et al. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol 2012;30:533-8. [PubMed]
- Del Mastro L, Levaggi A, Poggio F, et al. Role of temporary ovarian suppression obtained with GNRH analogue in reducing premature ovarian failure (POF) induced by chemotherapy in premenopausal cancer patients: a meta-analysis of randomized studies. ESMO Congress 2012, Ann Oncol 2012;23:Suppl 9 (abstract: 1551PD).
- Yang B, Shi W, Yang J, et al. Concurrent treatment with gonadotropin-releasing hormone agonists for chemotherapy-induced ovarian damage in premenopausal women with breast cancer: a meta-analysis of randomized controlled trials. Breast 2013;22:150-7. [PubMed]
- ISFP Practice Committee, Kim SS, Donnez J, et al. Recommendations for fertility preservation in patients with lymphoma, leukemia, and breast cancer. J Assist Reprod Genet 2012;29:465-8. [PubMed]
- Practice Committees of American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril 2013;99:37-43 [PubMed]
- Michaan N, Ben-David G, Ben-Yosef D, et al. Ovarian stimulation and emergency in vitro fertilization for fertility preservation in cancer patients. Eur J Obstet Gynecol Reprod Biol 2010;149:175-7. [PubMed]
- Sönmezer M, Türkçüoğlu I, Coşkun U, et al. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril 2011;95:2125.e9-11.
- Bedoschi GM, de Albuquerque FO, Ferriani RA, et al. Ovarian stimulation during the luteal phase for fertility preservation of cancer patients: case reports and review of the literature. J Assist Reprod Genet 2010;27:491-4. [PubMed]
- Oktay K. Further evidence on the safety and success of ovarian stimulation with letrozole and tamoxifen in breast cancer patients undergoing in vitro fertilization to cryopreserve their embryos for fertility preservation. J Clin Oncol 2005;23:3858-9. [PubMed]
- Oktay K, Buyuk E, Libertella N, et al. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol 2005;23:4347-53. [PubMed]
- Oktay K, Buyuk E, Davis O, et al. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod 2003;18:90-5. [PubMed]
- Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol 2008;26:2630-5. [PubMed]
- Chung K, Donnez J, Ginsburg E, et al. Emergency IVF versus ovarian tissue cryopreservation: decision making in fertility preservation for female cancer patients. Fertil Steril 2013;99:1534-42. [PubMed]
- Cao YX, Chian RC. Fertility preservation with immature and in vitro matured oocytes. Semin Reprod Med 2009;27:456-64. [PubMed]
- Fadini R, Dal Canto MB, Mignini Renzini M, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod Biomed Online 2009;19:343-51. [PubMed]
- von Wolff M, Donnez J, Hovatta O, et al. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy--a technique in its infancy but already successful in fertility preservation. Eur J Cancer 2009;45:1547-53. [PubMed]
- Donnez J, Dolmans MM. Cryopreservation and transplantation of ovarian tissue. Clin Obstet Gynecol 2010;53:787-96. [PubMed]
- Oktay K. Evidence for limiting ovarian tissue harvesting for the purpose of transplantation to women younger than 40 years of age. J Clin Endocrinol Metab 2002;87:1907-8. [PubMed]
- Newton H, Aubard Y, Rutherford A, et al. Low temperature storage and grafting of human ovarian tissue. Hum Reprod 1996;11:1487-91. [PubMed]
- Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med 2000;342:1919. [PubMed]
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004;364:1405-10. [PubMed]
- Meirow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med 2005;353:318-21. [PubMed]
- Radford JA, Lieberman BA, Brison DR, et al. Orthotopic reimplantation of cryopreserved ovarian cortical strips after high-dose chemotherapy for Hodgkin’s lymphoma. Lancet 2001;357:1172-5. [PubMed]
- Tryde Schmidt KL, Yding Andersen C, Starup J, et al. Orthotopic autotransplantation of cryopreserved ovarian tissue to a woman cured of cancer - follicular growth, steroid production and oocyte retrieval. Reprod Biomed Online 2004;8:448-53. [PubMed]
- Oktay K, Buyuk E, Rosenwaks Z, et al. A technique for transplantation of ovarian cortical strips to the forearm. Fertil Steril 2003;80:193-8. [PubMed]
- Oktay K, Economos K, Kan M, et al. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA 2001;286:1490-3. [PubMed]
- Meirow D, Raanani H, Brengauz M, et al. Results of one center indicate that transplantation of thawed ovarian tissue is effective. Repeated IVF reveals good egg quality and high pregnancy rate. Hum Reprod 2012;27:ii115-ii117.
- Kim SS, Lee WS, Chung MK, et al. Long-term ovarian function and fertility after heterotopic autotransplantation of cryobanked human ovarian tissue: 8-year experience in cancer patients. Fertil Steril 2009;91:2349-54. [PubMed]
- Kim SS, Radford J, Harris M, et al. Ovarian tissue harvested from lymphoma patients to preserve fertility may be safe for autotransplantation. Hum Reprod 2001;16:2056-60. [PubMed]
- Oktay K, Rodriguez-Wallberg K, Schover L. Preservation of fertility in patients with cancer. N Engl J Med 2009;360:2681-author reply 2682-3. [PubMed]
- Meirow D, Hardan I, Dor J, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod 2008;23:1007-13. [PubMed]
- Dolmans MM, Jadoul P, Gilliaux S, et al. A review of 15 years of ovarian tissue bank activities. J Assist Reprod Genet 2013;30:305-14. [PubMed]
- Sánchez-Serrano M, Novella-Maestre E, Roselló-Sastre E, et al. Malignant cells are not found in ovarian cortex from breast cancer patients undergoing ovarian cortex cryopreservation. Hum Reprod 2009;24:2238-43. [PubMed]
- Rosendahl M, Timmermans Wielenga V, Nedergaard L, et al. Cryopreservation of ovarian tissue for fertility preservation: no evidence of malignant cell contamination in ovarian tissue from patients with breast cancer. Fertil Steril 2011;95:2158-61. [PubMed]
- Azem F, Hasson J, Ben-Yosef D, et al. Histologic evaluation of fresh human ovarian tissue before cryopreservation. Int J Gynecol Pathol 2010;29:19-23. [PubMed]
- Fabbri R, Venturoli S, D’Errico A, et al. Ovarian tissue banking and fertility preservation in cancer patients: histological and immunohistochemical evaluation. Gynecol Oncol 2003;89:259-66. [PubMed]
- Bittinger SE, Nazaretian SP, Gook DA, et al. Detection of Hodgkin lymphoma within ovarian tissue. Fertil Steril 2011;95:803.e3-6.
- Loprinzi CL, Wolf SL, Barton DL, et al. Symptom management in premenopausal patients with breast cancer. Lancet Oncol 2008;9:993-1001. [PubMed]
- Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Hum Reprod Update 2009;15:587-97. [PubMed]
- Schover LR. Patient attitudes toward fertility preservation. Pediatr Blood Cancer 2009;53:281-4. [PubMed]
- Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol 2004;22:4174-83. [PubMed]
- Rippy EE, Karat IF, Kissin MW. Pregnancy after breast cancer: the importance of active counselling and planning. Breast 2009;18:345-50. [PubMed]
- Gracia CR, Jeruss JS. Lives in the balance: women with cancer and the right to fertility care. J Clin Oncol 2013;31:668-9. [PubMed]


