Serum KL-6 and surfactant protein-D as monitoring and predictive markers of interstitial lung disease in patients with systemic sclerosis and mixed connective tissue disease
Introduction
Interstitial lung disease (ILD) develops in more than half of the patients with systemic sclerosis (SSc) and is an important risk factor of mortality as is pulmonary hypertension (1,2). Moreover, patients with mixed connective tissue disease (MCTD) will likely suffer from ILD at some time (3), and these patients often have original features of SSc or develop SSc (4). Typically, the onset of SSc-ILD is insidious, with subtle clinical symptoms, which may explain why SSc-ILD is often diagnosed at an advanced stage, after extensive lung fibrosis is already present (5). In the treatment of SSc-ILD, although cyclophosphamide therapy can suppress the decline of pulmonary function over the short term, optimal treatment of SSc-ILD remains to be established (6). Corticosteroid has also been widely used to treat SSc-ILD, but given the lack of convincing benefit and the increased risk of SSc renal crisis, the indication for moderate- to high-dose corticosteroid therapy in SSc-ILD is limited (7,8). Therefore, the proper timing to begin medication for SSc-ILD is also unclear. Because no larger trials of therapies for MCTD have been performed, accurate treatment of MCTD-ILD remains unclear (9). Although the lung function of most patients with SSc/MCTD-ILD declines slightly, some patients suffer severe and subacute progressive deterioration of ILD and thus might require medical intervention (6-9). However, how to predict this group of patients remains difficult and unclear.
The serum levels of Krebs von den Lungen-6 (KL-6) and surfactant protein-D (SP-D), which are lung-specific proteins, have been shown to correlate with clinical manifestations of the extent and activity of pulmonary fibrosis and inflammations in idiopathic pulmonary fibrosis, nonspecific interstitial pneumonia (NSIP), cryptogenic organizing pneumonia, hypersensitivity pneumonia, and radiation pneumonitis (10-13). Previous reports of serum biomarkers for SSc-ILD showed that the presence of elevated KL-6 values was a poor prognostic factor for patients with SSc-ILD (14). We also reported that patients with SSc/MCTD-ILD with a KL-6 level ≥1,000 U/mL had a worse survival curve than those with KL-6 <1,000 U/mL (15). There are few reports of the usefulness of biomarkers (e.g., KL-6, SP-D, and SP-A) for the detection and monitoring of SSc-ILD (11,16-18). We hypothesized that serum biomarkers KL-6 and SP-D might be useful predictors of the progressive deterioration of ILD.
In the present study, we retrospectively investigated the correlation between the biomarkers of serum KL-6 and SP-D and pulmonary function tests (PFT) in patients with SSc/MCTD-ILD. Moreover, we evaluated whether these two biomarkers are predictors of the progressive deterioration of ILD.
Methods
Study sample
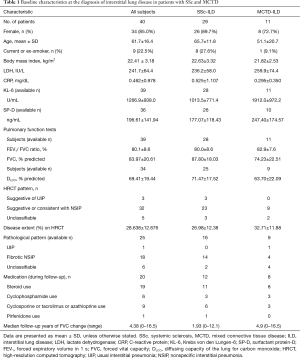
This study received approval from the institutional review board of Kanagawa Cardiovascular and Respiratory Center (no. 28-10). We retrospectively surveyed all patients who were diagnosed as having SSc/MCTD-ILD at Kanagawa Cardiovascular and Respiratory Center, Kanagawa, Japan, between March 1997 and July 2015. Three patients with SSc-rheumatoid arthritis overlapping syndrome and one patient with SSc/dermatomyositis and SSc-ILD were excluded from this study. The patient cohort was already the subject of a previous study focusing on interstitial pneumonia with SSc-related autoantibody (15). SSc and MCTD were diagnosed when patients fulfilled the established criteria (19-21). Two patients developed manifestations of SSc during the follow-up period, and these patients were also included as SSc-ILD subjects. Baseline clinical measurements were obtained within one month of the initial diagnosis of ILD at our hospital. The baseline characteristics of the 29 SSc-ILD patients and 11 MCTD-ILD patients are summarized in Table 1. Serum KL-6 concentrations were measured with commercially available ELISA kits (EIDIA Co., Japan) following the manufacturer’s instructions. Serum SP-D concentrations were also measured with commercially available ELISA kits (Yamasa Co., Japan).
Full table
Radiological analysis of interstitial pneumonia was classified as presenting a HRCT pattern either “suggestive or consistent with NSIP” or “suggestive of UIP (usual interstitial pneumonia)” (22,23). Disease extent (%) on HRCT was calculated by a three-dimensional computer-aided system (i.e., Gaussian histogram normalized correlation) of CT images (24,25). Pathological analysis was classified according to the current classification of idiopathic interstitial pneumonias by two pulmonary pathologists (T.T. and K.O.) (23).
Statistical analysis
First, the correlations between disease extent (%) on HRCT; PFT results of forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and diffusing capacity of the lung for carbon monoxide (DLCO); and the biomarkers of serum levels of KL-6 and SP-D were analyzed using Pearson correlation and Spearman’s rank correlation coefficient. If a patient’s available data indicated that serum levels of KL-6 were repeatedly obtained within 1 month of PFT during the follow-up period, the correlation of changes in KL-6 with changes in FVC from the initial to final measurement were also analyzed. Second, the correlation of serum KL-6 and SP-D with PFT results was analyzed by multivariate logistic regression analysis for the 22 patients with SSc/MCTD-ILD in whom the change in FVC from the initial to second measurement could be calculated. Third, because 8 of these 22 patients had a decline in FVC of more than 0.08 L/year, we thought that with further analysis, this value could be parsed as a cut-off level for the prediction of FVC decline. We then performed univariate and multivariate logistic regression analyses using the variable of FVC decline of >0.08 L/year to identify significant predictors of FVC decline in these patients. The predictive performance was then evaluated by calculating the area under the curve (AUC) of the receiver-operating characteristic curve (ROC). We considered P<0.05 to represent statistical significance in all analyses. All data were analyzed with SAS version 9.4 (SAS Institute Inc.).
Results
Patient characteristics
We identified 40 subjects of whom 29 were patients with SSc-ILD and 11 were patients with MCTD-ILD (Table 1). In both groups, more patients with SSc/MCTD-ILD were women, never-smokers, and had radiological and pathological findings of NSIP.
Correlations between biomarkers (KL-6 and SP-D), PFT (FVC and DLCO), and disease extent on HRCT
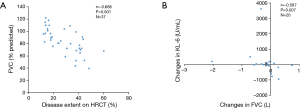
Although serum levels of KL-6 at the diagnosis of interstitial pneumonia did not correlate significantly with FVC (% predicted) and (r=−0.094, P=0.573) (Figure 1A), the serum levels of KL-6 did correlate significantly with DLCO (% predicted) and disease extent on HRCT (DLCO: r=−0.345, P=0.046; disease extent: r=0.418, P=0.010) (Figure 1B,C). However, serum levels of SP-D did not correlate significantly with FVC (% predicted) (r=−0.055, P=0.749) (Figure 1D), DLCO (% predicted) (r=−0.067, P=0.714), and disease extent on HRCT (r=0.160, P=0.367). FVC (% predicted) also correlated significantly with disease extent on HRCT (r=−0.668, P<0.001) (Figure 2A), and the changes in serum levels of KL-6 were significantly related to the changes in FVC from the initial to final measurement at a median of 1.9 (range, 0.3–16.5) years (r=−0.587, P=0.007) (Figure 2B).

Predictive factors of FVC change
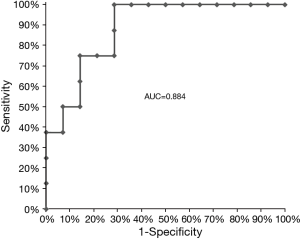
We analyzed predictors of FVC change from the initial to second measurement [median of 6 (range, 3–35) months] by logistic regression analysis by using explanatory variables such as KL-6, SP-D, FEV1, FEV1/FVC ratio, FVC, FVC (% predicted), DLCO, and DLCO (% predicted) (Table 2). No predictive factors were revealed by univariate analysis at significance levels below 5%. However, the analysis suggested that the act of matching factors such as KL-6, SP-D, FVC, DLCO, and DLCO (% predicted) was useful for predicting FVC changes by multivariate analysis. Therefore, the variables that achieved a modest level of statistical significance (P<0.15), based on the backward selection method, were SP-D, FVC, DLCO, and DLCO (% predicted) (Table 3). As a second step, we investigated prediction accuracy of a decline in FVC of >0.08 L/year using these variables and ROC analysis (Figure 3). The AUC of this curve was 0.884.

Full table

Full table
Predictive score
We considered a decline in FVC of >0.08 L/year on the ROC curve to be the optimal cut-off point by the Youden Index method (26) and then determined the optimal cut-off predictive score to be 0.398290 with the following formula:
The sensitivity, specificity, positive predictive value, and negative predictive value of this cut-off value were 75.0%, 85.7%, 75.0%, and 85.7%, respectively.
Case presentation
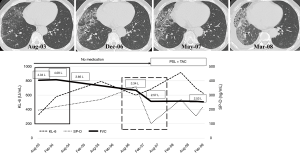
A 65-year-old man with SSc-ILD had a normal KL-6 level (329 U/mL) and slightly elevated SP-D (195 ng/mL) when he visited at our hospital in August 2003 (square bordered by solid line in Figure 4). His FVC at the first visit was within normal range at 4.04 L, and transthoracic echocardiography did not show apparent pulmonary hypertension or abnormal cardiac function. Chest HRCT showed a reticular shadow and ground-glass opacities predominantly in the right lower lung. He was asymptomatic and then received follow-up observation with no medication. In December 2006, the lesion extended and traction bronchiectasis appeared on the chest HRCT and then his FVC was slightly decreased, the SP-D level was elevated at 359.6 ng/mL, and he underwent video-assisted surgical lung biopsy. Pathological diagnosis of the lesion was fibrotic NSIP. Afterwards, although radiological deterioration was slight in May 2007, his KL-6 level increased to 685 U/mL, and his FVC had deteriorated to 2.57 L compared with December 2006 (rise in KL-6 level of about 100 U/mL and decline in FVC of 770 mL over 5 months) (square bordered by dashed line). At the same time, his SP-D was stabilized within normal range at 102.3 ng/mL. Afterwards, although chest HRCT showed gradually deterioration of disease extent and pleural effusion, he was started on prednisolone and tacrolimus, and then, his radiological changes, FVC, and SP-D stabilized.
Discussion
In the present study, serum KL-6 correlated positively with (DLCO) (% predicted) and disease extent on HRCT, and the changes in serum levels of KL-6 were significantly related to the changes in FVC in SSc/MCTD-ILD. On the other hand, our results suggested that a higher serum level of SP-D was a significant predictor of potential FVC decline in patients with SSc/MCTD-ILD according to the predictive score by multivariate logistic regression analysis and calculation of the ROC AUC.
First, our result that serum KL-6 level correlated inversely with (DLCO) (% predicted), which was the same kind of previous results (18,27,28). It is noteworthy that serum KL-6 at the initial visit correlated positively with disease extent on HRCT in SSc/MCTD-ILD. Sakamoto et al. reported similar results in patients with fibrotic NSIP (12). Our subjects predominantly had NSIP, and this might have affected the positive correlation results. Because a recently published expert opinion report highlighted FVC as a core outcome of chronic ILD (29), originally, we expected to directly prove a significant correlation between serum KL-6 and FVC. However, our study could not show significant results at this point, possibly due to its small sample size. Moreover, the changes in serum levels of KL-6 were significantly related to the changes in FVC. Yanaba et al. previously reported that KL-6 levels in 4 patients increased rapidly, in parallel with the progression of SSc-ILD, whereas those in 4 other patients with stable SSc-ILD activity remained stable during follow-up (17). Our results also supported the change in the serum level of KL-6 as a useful monitoring tool of ILD activity as the FVC declines in SSc/MCTD patients.
Second, in our study, the serum level of SP-D was a significant predictor of FVC decline in SSc/MCTD-ILD by multivariate logistic regression analysis. Previously, only one report showed that an increased concentration of SP-D was more closely associated with decreased vital capacity in SSc patients than was that of KL-6 (18). In our case presentation, the serum level of SP-D at the initial visit was low, and the FVC was relatively stable for about 3 years. However, SP-D increased by 1.84 times that at the initial visit, and soon thereafter, the patient’s FVC rapidly decreased. In contrast, the serum level of KL-6 was not found to be a predictive factor of FVC decline in this case. Therefore, higher serum levels of SP-D appear to be a predictor of the progressive deterioration of ILD.
Medical treatment of SSc-ILD in general has been unsatisfactory (30). SSc-ILD was reported to progress much more frequently in the first 4 years, and then a certain number of patients showed stabilization of ILD progression with or without medical intervention (31). In patients with MCTD-ILD, FVC was also similarly reported to be slightly reduced at baseline but remained stable after 10 years (9). However, clinicians should be careful of the timing of medical intervention during follow-up because some patients have severe and subacute progressive deterioration of ILD (6-9). Our analysis showed that when the serum level of SP-D in the patients with SSc/MCTD-ILD increased to a higher level during follow-up, their FVC could rapidly decline, and then these patients frequently required medical examination and/or medical intervention. High levels of serum KL-6, older age, lower FVC, lower DLCO, and the presence of honeycombing on HRCT are reported to be a poor prognostic factors of SSc-ILD (14). Therefore, it is necessary to exercise caution when caring for SSc/MCTD-ILD patients with these factors. In addition, because of a small size analysis, whether intervention of the medical treatment effected the correlation between PFT and KL-6/SP-D levels or not could have not been satisfactory evaluated. However, the patient presented as case presentation in our text, who were thought to be positive correlation with these parameters during follow-up. We believe that serum KL-6 and SP-D can be useful predictive tools, particularly in the patient having the above poor prognostic factors.
Our study has limitations. First, we included both patients with MCTD and those with SSc. As mentioned in previous studies, most patients with MCTD-ILD have characteristics of SSc and ultimately develop the disease (4,15,32,33). Moreover, the progression of ILD in patients with SSc and MCTD exhibits similar disease behavior, which often stabilizes after a couple of years (9,31). Therefore, ILD associated with MCTD is thought to be a similar disease entity to that of SSc-ILD. Second, because this was a retrospective single-center study with a small sample size, the level of confidence is reduced, and thus, further studies with a larger sample size are needed.
Our study suggested that changes in serum levels of KL-6 were significantly related to the changes in FVC in patients with SSc/MCTD-ILD, which indicates that serum KL-6 could be a useful monitoring tool of ILD activity as the FVC changes in these patients. In contrast, the serum level of SP-D was a significant predictor of FVC decline in these patients. We believe that the combination of KL-6 and SP-D will be helpful in the evaluation of SSc/MCTD-ILD disease activity and the timing of medical interventions.
Acknowledgements
We offer our sincerest thanks to Drs. Shigeru Komatsu, Takeshi Shinohara, and Shinko Sadoyama of the Department of Respiratory Medicine, Kanagawa Cardiovascular and Respiratory Center, for their handling of the diagnosis and treatment of the patients in our study, and to Hideyo Oda of Medical Toukei Co., Ltd. for his advice on statistical analysis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study received approval from the institutional review board of Kanagawa Cardiovascular and Respiratory Center (No. 28-10).
References
- Silver RM. Scleroderma. Clinical problems. The lungs. Rheum Dis Clin North Am 1996;22:825-40. [Crossref] [PubMed]
- Hasegawa M, Asano Y, Endo H, et al. Investigation of prognostic factors for skin sclerosis and lung function in Japanese patients with early systemic sclerosis: a multicentre prospective observational study. Rheumatology (Oxford) 2012;51:129-33. [Crossref] [PubMed]
- Fagundes MN, Caleiro MT, Navarro-Rodriguez T, et al. Esophageal involvement and interstitial lung disease in mixed connective tissue disease. Respir Med 2009;103:854-60. [Crossref] [PubMed]
- Smolen JS, Steiner G. Mixed connective tissue disease: to be or not to be? Arthritis Rheum 1998;41:768-77. [Crossref] [PubMed]
- Hoffmann-Vold AM, Aaløkken TM, Lund MB, et al. Predictive value of serial high-resolution computed tomography analyses and concurrent lung function tests in systemic sclerosis. Arthritis Rheumatol 2015;67:2205-12. [Crossref] [PubMed]
- Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006;354:2655-66. [Crossref] [PubMed]
- Hoyles RK, Ellis RW, Wellsbury J, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum 2006;54:3962-70. [Crossref] [PubMed]
- Steen VD, Medsger TA Jr. Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum 1998;41:1613-9. [Crossref] [PubMed]
- Gunnarsson R, Hetlevik SO, Lilleby V, et al. Mixed connective tissue disease. Best Pract Res Clin Rheumatol 2016;30:95-111. [Crossref] [PubMed]
- Kohno N, Kyoizumi S, Awaya Y, et al. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest 1989;96:68-73. [Crossref] [PubMed]
- Takahashi H, Kuroki Y, Tanaka H, et al. Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis. Am J Respir Crit Care Med 2000;162:258-63. [Crossref] [PubMed]
- Sakamoto K, Taniguchi H, Kondoh Y, et al. Serum KL-6 in fibrotic NSIP: Correlations with physiologic and radiologic parameters. Respir Med 2010;104:127-33. [Crossref] [PubMed]
- Okada F, Ando Y, Honda K, et al. Comparison of pulmonary CT findings and serum KL-6 levels in patients with cryptogenic organizing pneumonia. Br J Radiol 2009;82:212-8. [Crossref] [PubMed]
- Winstone TA, Assayag D, Wilcox PG, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest 2014;146:422-36. [Crossref] [PubMed]
- Yamakawa H, Hagiwara E, Kitamura H, et al. Clinical Features of Idiopathic Interstitial Pneumonia with Systemic Sclerosis-Related Autoantibody in Comparison with Interstitial Pneumonia with Systemic Sclerosis. PLoS One 2016;11:e0161908. [Crossref] [PubMed]
- Asano Y, Ihn H, Yamane K, et al. Clinical significance of surfactant protein D as a serum marker for evaluating pulmonary fibrosis in patients with systemic sclerosis. Arthritis Rheum 2001;44:1363-9. [Crossref] [PubMed]
- Yanaba K, Hasegawa M, Hamaguchi Y, et al. Longitudinal analysis of serum KL-6 levels in patients with systemic sclerosis: association with the activity of pulmonary fibrosis. Clin Exp Rheumatol 2003;21:429-36. [PubMed]
- Yanaba K, Hasegawa M, Takehara K, et al. Comparative study of serum surfactant protein-D and KL-6 concentrations in patients with systemic sclerosis as markers for monitoring the activity of pulmonary fibrosis. J Rheumatol 2004;31:1112-20. [PubMed]
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737-47. [Crossref] [PubMed]
- Sharp GC, Irvin WS, May CM, et al. Association of antibodies to ribonucleoprotein and Sm antigens with mixed connective-tissue disease, systematic lupus erythematosus and other rheumatic diseases. N Engl J Med 1976;295:1149-54. [Crossref] [PubMed]
- Cappelli S, Bellando Randone S, et al. "To be or not to be," ten years after: evidence for mixed connective tissue disease as a distinct entity. Semin Arthritis Rheum 2012;41:589-98. [Crossref] [PubMed]
- American Thoracic Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Iwasawa T, Kato S, Ogura T, et al. Low-normal lung volume correlates with pulmonary hypertension in fibrotic idiopathic interstitial pneumonia: computer-aided 3D quantitative analysis of chest CT. AJR Am J Roentgenol 2014;203:W166-73. [Crossref] [PubMed]
- Iwasawa T, Kanauchi T, Hoshi T, et al. Multicenter study of quantitative computed tomography analysis using a computer-aided three-dimensional system in patients with idiopathic pulmonary fibrosis. Jpn J Radiol 2016;34:16-27. [Crossref] [PubMed]
- Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J 2005;47:458-72. [Crossref] [PubMed]
- Yamane K, Ihn H, Kubo M, et al. Serum levels of KL-6 as a useful marker for evaluating pulmonary fibrosis in patients with systemic sclerosis. J Rheumatol 2000;27:930-4. [PubMed]
- Bonella F, Volpe A, Caramaschi P, et al. Surfactant protein D and KL-6 serum levels in systemic sclerosis: correlation with lung and systemic involvement. Sarcoidosis Vasc Diffuse Lung Dis 2011;28:27-33. [PubMed]
- Saketkoo LA, Mittoo S, Huscher D, et al. Connective tissue disease related interstitial lung diseases and idiopathic pulmonary fibrosis: provisional core sets of domains and instruments for use in clinical trials. Thorax 2014;69:428-36. [Crossref] [PubMed]
- Steen VD, Conte C, Owens GR, et al. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum 1994;37:1283-9. [Crossref] [PubMed]
- Wells AU. Interstitial lung disease in systemic sclerosis. Presse Med 2014;43:e329-43. [Crossref] [PubMed]
- Nimelstein SH, Brody S, McShane D, et al. Mixed connective tissue disease: a subsequent evaluation of the original 25 patients. Medicine (Baltimore) 1980;59:239-48. [Crossref] [PubMed]
- Aringer M, Steiner G, Smolen JS. Does mixed connective tissue disease exist? Yes. Rheum Dis Clin North Am 2005;31:411-20. v. [Crossref] [PubMed]


