Role of bronchoprovocation tests in identifying exercise-induced bronchoconstriction in a non-athletic population: a pilot study
Introduction
Exercise-induced bronchoconstriction (EIB) is a common condition, especially in the athletic and military populations, and its prevalence is increasing worldwide (1,2). In the military setting, EIB is associated with military training failure, and has been implicated in death and army discharges (3).
Testing for EIB is important in the context of Singapore’s military conscription. Most commonly used testing in our clinical setting is exercise challenge test (ET), but it can be difficult to implement (4), and may produce false-negative results if performed only once (5). Generally, diagnostic tests for exercise induced bronchoconstriction are divided into direct and indirect challenges. Direct challenges involve administering a single agent such as methacholine which acts directly on the smooth muscle receptors to precipitate contraction. Indirect challenges involve introduction of exercise or other agents such as hypertonic saline (HS) or mannitol to provoke cellular mediator release (including prostaglandins, leukotrienes and histamine) which causes airway smooth muscle contraction. Recent guidelines recommend indirect challenges over direct challenges (6), however choice of challenge test is dependent on many factors including availability of equipment (treadmill machine for ET, ultrasonic nebulizers for HS, balloon and gas cylinders for eucapnic voluntary hyperpnea test), availability of the bronchoprovocative agent (methacholine, HS or mannitol), availability of the technical expertise in the laboratory, cost and lastly physician preference.
We sought to compare sensitivity, specificity, accuracy and patient preference of HS challenge to ET in a population of non-athletic participants who had symptoms of EIB. Our secondary objective was to compare retrospectively retrieved MCT results performed within one year of recruitment, and evaluate its agreement with the HS and ET results.
Methods
Study design
Participants were recruited from the respiratory outpatient clinic of Singapore General Hospital, between May 2012 and February 2015. Inclusion criteria were met if participants had exercise-induced dyspnoea and were corticosteroid-naive. Use of salbutamol (as needed) was permitted. Subjects were excluded if they had presence of orthopaedic problems limiting exercise. The participants’ history of asthma, prior to study recruitment was recorded. Eligible subjects then underwent both ET and HS tests on separate days not more than 2 weeks apart. All except 1 subject underwent ET first, followed by HS.
Measurements
ET
Subjects undergoing ET had a baseline spirometry performed before exercising on a treadmill in an air-conditioned room with ambient temperature of 20 degrees celsius and a relative humidity of 43%±1% according to ATS recommendations (4). Treadmill speed and grade were progressively advanced within the first 2–3 minutes until heart rate reached 85% of predicted maximum (calculated as 220-age in years). The test was terminated when the subject had exercised at the target heart rate for 5 minutes and post-test spirometry after 5 minutes was performed. A positive test was defined as a reduction in post-exercise forced expiratory volume in 1 second (FEV1) of 10% or more compared to the baseline (4). If there was no reduction in FEV1 of 10% or more, spirometry was repeated at 5 minute intervals (5, 10, 15 and 20 minutes after exercise). Three repeatable flow-volume loops were performed at each interval period.
HS testing
Subjects were made to inhale HS at 4.5% for increasing periods (0.5, 1, 2, 4 and 8 minutes) at intervals of 2 minutes, totaling 15 minutes and 30 seconds. FEV1 measurements were taken 1 minute after the end of each inhalation. Three repeatable flow-volume loops were performed after each inhalational period. Reduction in FEV1 of 15% or more compared to the baseline indicated a positive test and the test was terminated. The test was also terminated when the cumulative inhalation time of 15.5 min had been achieved without significant reduction in FEV1. The result was presented as the provocative dose of saline inducing a 15% fall in FEV1 (PD15) (7).
Methacholine challenge test (MCT)
MCT results performed within one year of ET and HS were retrospectively retrieved and included in the analysis. Methacholine challenge was performed using the five-breath dosimeter protocol (4). Subjects inhaled methacholine solution at increasing concentrations (0.25, 1, 4, 16 mg/mL), until a positive result was obtained or the highest dose of methacholine (16 mg/mL) had been delivered. A positive MCT was defined as a provocative dose of methacholine inducing a 20% reduction in FEV1 (PC20) <16 mg/mL.
At the end of each test, subjects were asked to fill in 2 separate multiple choice questionnaires to indicate their preferred test (HS or ET) as well as level of difficulty with each test.
Statistical analysis
Statistical analysis was performed using SPSS (Version 21.0). Confidence intervals of 95% were reported, and all tests were two-sided. Continuous variables were expressed as mean ± standard deviation. Correlations between variables were examined using Pearson’s correlation coefficients. HS responses were log-transformed for calculation of geometric means. Kappa statistics are indices of inter-rater reliability used to measure the level of agreement, performed using SPSS. Sensitivity, specificity, predictive values and accuracy were calculated based on standard definitions, with P<0.05 implying statistical significance.
Results
Twenty-seven participants were included in the study. The baseline characteristics of the subjects are shown in Table 1. Subjects with and without EIB were not significantly different in their demographics, except for a higher proportion of allergic rhinitis among the subjects without EIB.
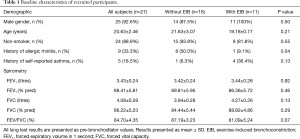
Full table
The prevalence of EIB in this cohort was 40.7% (11/27) using ET as the ‘gold standard’. Sixteen subjects (59.2%) tested positive for HS and thirteen (61.9%) had tested positive for MCT. Nine subjects (42.9%) tested positive for all three tests (HS, ET and MCT).The performance of all three tests is shown in Table 2.
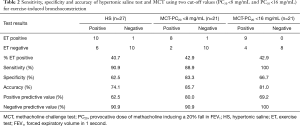
Full table
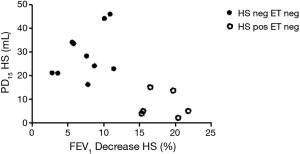
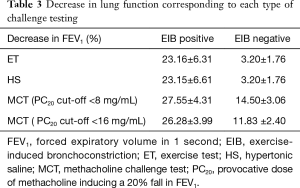
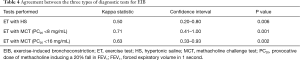
Our pilot study demonstrated a high sensitivity of 90.9% for the HS challenge test with a moderate specificity of 62.5% to diagnose EIB in a non-athletic population. There were six subjects that tested negative for ET but positive for HS. As shown (Figure 1), the PD15 for these six subjects is lower compared to those who tested negative to both ET and HS. Table 3 shows the decrease in lung function corresponding to each type of challenge testing, which was very similar for HS and ET. In addition, Pearson’s correlation demonstrated a significant and positive relationship between the reactivity to HS and the post-exercise fall in FEV1 (r=0.702, P<0.001). Furthermore, the kappa statistic of agreement between HS and ET showed moderate agreement (Kappa =0.50, Table 4). In the post-test survey, there was no clear patient preference for either test, with 15 subjects (55.6%) preferring ET over HS.
Full table
Full table
MCT was measured within one year of enrollment, with a median time window of 9 days (interquartile range 0–14 days). In a secondary analysis, MCT demonstrated a higher specificity of 83.3% using a cut-off value of PC20 <8 mg/mL as compared to 66.7% for PC20 <16 mg/mL to detect EIB. Negative predictive values (NPV) were 90.9% and 100% for both MCT cut-offs respectively. MCT had substantial agreement with ET (Kappa statistic 0.71 for PC20 <8 mg/mL; Kappa statistic 0.63 for PC20 <16 mg/mL, Table 3).
Discussion
The primary aim of this study was to determine the diagnostic performance of HS challenge test for diagnosing EIB, determined by ET, in a non-athletic population. Our pilot study demonstrated high sensitivity of 90.9% for HS challenge test with a lower specificity of 62.5%. Subjects had no preference for HS test over the ET. In a secondary analysis, retrospectively collected MCT results demonstrated higher specificity of 83.3% using a cut-off value of PC20 <8 mg/mL, and 66.7% for PC20 <16 mg/mL, with NPVs of 90.9% and 100% respectively.
Recently published joint AAAAI and ACAAI Task Force statement had recommended indirect challenges (exercise, EVH or mannitol) over direct challenges such as MCT for the diagnosis of EIB (6). Strenuous exercise creates a hyperosmolar environment by introducing dry air in the airway with compensatory water loss, leading to transient osmotic change on the airway surface (8). The hyperosmolar environment leads to mast cell degranulation with release of mediators, including histamine, tryptase, and prostaglandins. In addition, eosinophils can also be activated producing further mediators, including leukotrienes. Therefore, indirect challenges are more specific in reflecting bronchial hyperresponsiveness caused by exercise and potentially the need for inhaled corticosteroids (6).
HS testing performed well, with a NPV of 90.9%, and sensitivity of 90.9%. Previous studies evaluating HS have been performed in the paediatric population (9) or subjects with asthma (10), and have demonstrated moderate to high sensitivity and specificity. Depending on the clinical indication, either high sensitivity or specificity can be useful. For the screening of military conscripts, a high sensitivity would be more useful to reduce inadvertent inclusion of subjects with asthma or EIB into military service without proper treatment and control of underlying airway inflammation.
Our secondary analysis demonstrated that MCT performed exceptionally well compared to ET, with sensitivity reaching 100%. This result is unprecedented, especially as the five-breath dosimeter protocol that was used for the MCT protocol in this study is associated with more false-negative testing compared to the tidal breathing technique (11), which is used by the International Olympic Committee (positive test defined as PC20 ≤4 mg/mL using tidal breathing technique) (12). One likely explanation for this finding could be due to variability of laboratory ET in diagnosing EIB as shown in a previous study from Anderson’s group (5). They challenged 373 subjects twice using treadmill ET and found that the agreement between results was only 76.1%. Another explanation could be that this finding is related to a number of previously undiagnosed asthmatics with overlapping EIB in our study population. Similar to the heterogeneity of asthma, EIB has separate phenotypes, and it has previously been shown that up to 90% of asthmatic subjects could have EIB (13). It is possible that the MCT is detecting the subjects from the asthmatic group, whereas the international recommendations were based on studies largely recruiting elite athletes and hence having different pathogenetic mechanisms. Prior studies carried out using MCT in asthmatic subjects had variable results for sensitivity ranging from 51–87.7% (10,14,15), which was slightly lower than our documented sensitivities (Table 2). These differences could possibly stem from different PC20 cut-off values used in each study as we have also documented that using PC20 <8 mg/mL and PC20 <16 mg/mL, there were differences in sensitivities (88.9% vs. 100%) and specificities (83.3% vs. 66.7%) respectively.
The major limitation of the study was that MCT data were collected retrospectively within one year from inclusion, and moreover not all subjects underwent MCT. The retrospective review of MCT confines the interpretation of the agreement between MCT and ET. However, previous studies have suggested that MCT is reproducible in symptomatic asthmatic subjects ranging from one week (16) to one year (17). In addition, most of the patients underwent MCT within 1 month of inclusion (except for 2 patients). Other limitations included the small sample size as it was a pilot study and the lack of sequential testing to evaluate the reproducibility of our results.
In conclusion, this study shows that HS in non-athletes displays moderate agreement with ET and is a potential surrogate screening test due to its high NPV. However, ET test may have to be repeated if the first test was negative as EIB may not be confidently ruled out (5). The agreement that has been demonstrated between MCT performed within a year of inclusion and the ET can come in useful for predicting EIB in centers that have limited access to indirect testing. Even though HS and MCT demonstrated good NPVs, further studies with protocols incorporating multiple challenges of ET, HS and MCT in sequence would be useful to evaluate reproducibility of results.
Acknowledgements
The authors would like to thank the Pulmonary Function Laboratory staff in SGH for their expertise in performing the pulmonary function tests, as well as Ms. Xiaohui Xin and Mr. Edmund Teo, biostatisticians from Singapore General Hospital Academic Clinical Programme (Medicine), for their statistical advice.
Funding: This study was funded by the SingHealth Start-up Grant (SHF/FG499S/2011).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by SingHealth Centralised Institutional Review Board (No. CIRB 2011/571/C) and written informed consent was obtained from all patients.
References
- Holzer K, Brukner P. Screening of athletes for exercise-induced bronchoconstriction. Clin J Sport Med 2004;14:134-8. [Crossref] [PubMed]
- Carlsen KH, Anderson SD, Bjermer L, et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy 2008;63:387-403. [Crossref] [PubMed]
- Stocks J, Tripp M, Lin T. Methacholine challenge is insufficient to exclude bronchial hyper-responsiveness in a symptomatic military population. J Asthma 2014;51:886-90. [Crossref] [PubMed]
- Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 2000;161:309-29. [PubMed]
- Anderson SD, Pearlman DS, Rundell KW, et al. Reproducibility of the airway response to an exercise protocol standardized for intensity, duration, and inspired air conditions, in subjects with symptoms suggestive of asthma. Respir Res 2010;11:120. [Crossref] [PubMed]
- Weiler JM, Brannan JD, Randolph CC, et al. Exercise-induced bronchoconstriction update-2016. J Allergy Clin Immunol 2016;138:1292-1295.e36. [Crossref] [PubMed]
- Sterk PJ, Fabbri LM, Quanjer PH, et al. Standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Eur Respir J 1993;6 Suppl 16:53-83. [Crossref] [PubMed]
- Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is .. J Allergy Clin Immunol 2000;106:453-9. [Crossref] [PubMed]
- Riedler J, Reade T, Dalton M, et al. Hypertonic saline challenge in an epidemiologic survey of asthma in children. Am J Respir Crit Care Med 1994;150:1632-9. [Crossref] [PubMed]
- Choi IS, Chung SW, Koh YI, et al. Airway hyperresponsiveness to hypertonic saline as a predictive index of exercise-induced bronchoconstriction. Korean J Intern Med 2005;20:284-9. [Crossref] [PubMed]
- Todd DC, Davis BE, Hurst TS, et al. Dosimeter methacholine challenge: comparison of maximal versus submaximal inhalations. J Allergy Clin Immunol 2004;114:517-9. [Crossref] [PubMed]
- International Olympic Medical Commission. Beta2 adrenoceptor agonists and the Olympic Games in Beijing. 2008.
- Weiler JM, Anderson SD, Randolph C, et al. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Ann Allergy Asthma Immunol 2010;105:S1-47. [Crossref] [PubMed]
- Anderson SD, Charlton B, Weiler JM, et al. Comparison of mannitol and methacholine to predict exercise-induced bronchoconstriction and a clinical diagnosis of asthma. Respir Res 2009;10:4. [Crossref] [PubMed]
- Miedinger D, Mosimann N, Meier R, et al. Asthma tests in the assessment of military conscripts. Clin Exp Allergy 2010;40:224-31. [Crossref] [PubMed]
- Higgins BG, Britton JR, Chinn S, et al. Comparison of histamine and methacholine for use in bronchial challenge tests in community studies. Thorax 1988;43:605-10. [Crossref] [PubMed]
- Newill CA, Eggleston PA, Prenger VL, et al. Prospective study of occupational asthma to laboratory animal allergens: stability of airway responsiveness to methacholine challenge for one year. J Allergy Clin Immunol 1995;95:707-15. [Crossref] [PubMed]


