The role of BioGlue in thoracic surgery: a systematic review
Introduction
Thoracic surgery has significantly evolved over the last 20 years achieving low mortality and morbidity rates. However, complications following thoracic operations still remain an issue and notably affect the postoperative course, with an added economical burden to health care systems (1). Amongst them, alveolar air leaks (AAL) and bronchopleural fistulae (BPF) are considered quite challenging in terms of management and lead to prolonged chest tube drainage, LOS and increased risk of pleural infections (2,3).
Previous studies have shown that prolonged air leak, defined as seven days or more in duration, occur with high incidence after major thoracic operations (4). On the other hand, development of BPF, although presenting with a lower incidence, is associated with higher mortality rates (5). This has led to the development of several techniques to combat such complications. These include, but are not limited to, pleurodesis, placement of additional drains, thoracotomy and manual closure of the bronchial stump, intrathoracic muscle or omental flap transposition and use of different types of sealants, such as fibrin glue and Progel® (Neomend, Inc., Irvine, CA, USA) (6-8).
BioGlue® (CryoLife International Inc., Kennesaw, GA, USA) is an adhesive applied in several surgical specialties and its use has been documented in thoracic surgery as well. It consists of purified bovine serum albumin (BSA) and glutaraldehyde and produces a stable, solid medium after these two components bind to each other (9,10). In the present study, we systematically reviewed the literature regarding BioGlue in order to analyze its role in thoracic surgery and especially its application in the treatment of AAL and BPF.
Methods
Search strategy, data sources and eligibility criteria
The systematic review was conducted in accordance to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines (Table S1) (11). The study protocol was discussed and agreed by all authors. A systematic literature search was performed using the Medline database and Cochrane library—Cochrane Central Register of Controlled Trials (CENTRAL) through July 2016; the terms “bioglue”, “albumin-Glutaraldehyde tissue adhesive” and “sealants” were combined with the terms “thoracic”, “lung”, “air-leak” and “bronchial fistula”.
Full table
Two authors (DI Tsilimigras, A Antonopoulou) worked independently and screened all available studies. The references of all relevant studies were manually assessed to avoid missing any available data.
The inclusion criteria consisted of: studies reporting on thoracic surgery operations and use of BioGlue in thoracic surgical procedures.
The exclusion criteria consisted of: non-English language studies, non-human population, studies on surgical specialties other than Thoracic surgery, reviews and meta-analyses and sealants other than BioGlue.
Data extraction and analysis
Two authors (DI Tsilimigras, A Antonopoulou) working separately extracted the data from the eligible studies and subsequently cross-checked the results. Any discrepancies were resolved following discussion and consensus amongst all participating authors. Variables that were extracted included: general study characteristics (author, year of publication, number of patients), patients demographics (sex, age, country), type of surgical procedure, indication for using BioGlue, concomitant use of BioGlue with other device/glue, duration of air leak, duration of chest tube drainage, LOS, complications and associated recurrence-free interval (RFI).
Results
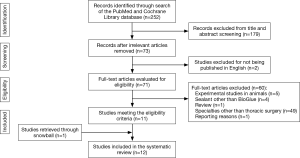
The search algorithm yielded 252 studies and following screening, 73 studies were retrieved for full-text review. Twelve studies finally met our inclusion criteria and were included in the present systematic review (9,10,12-21). One study, although relevant, was eventually excluded due to reporting reasons (22) (Figure 1).
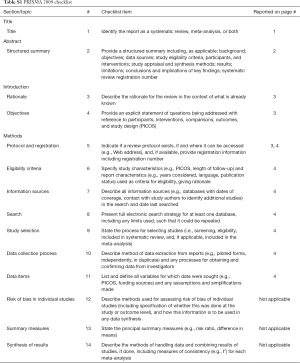
Features and demographics of the included studies are summarized in Table 1. Amongst them, seven articles were from UK, two from Greece, two from the USA and one from Australia. Overall, 194 patients were allocated with a 1:9 male to female ratio. Although BioGlue was used in a variety of Thoracic surgical procedures, the indications for application were mainly prolonged AAL and management of BPF. Four studies targeted the first indication (9,14-16), six studies the second (12,13,18-21), while in two studies both indications were analyzed (10,17) and these are presented separately. Regarding the prevention of air leak, BioGlue was mainly utilized at the time of initial operation (9,14-16), except for six cases (172/178), in whom prolonged air leak after primary procedure necessitated its application (10). On the other hand, BPF and lymphatic leak treatment was associated with secondary interventions in all but one case for each group [BPF: 13 (92.9%), lymphatic leak: 1 (50%)] following major thoracic surgery procedures (10,12,13,17-21).
Full table
Prevention of prolonged air leak
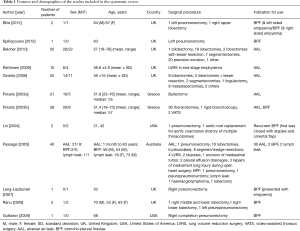
The four studies (9,14-16), referring exclusively to prevention of prolonged AAL, are summarized in Table 2. In all cases, the application of BioGlue was performed at the time of initial operation. The indication for its use was failure to control the air leak by conventional means such as sutures, diathermy and stapling (9,14) as well as prevention of air leak after lung volume reduction surgery (LVRS) (15) and bullectomy (16).
Full table
In a prospective randomized controlled trial, Belcher et al. compared BioGlue and Vivostat for the control of AAL and showed no statistically significant difference regarding duration of air leak, duration of intercostal drainage, LOS and incidence of complications (14) (Table 2).
In another pilot randomized controlled trial, patients undergoing LVRS were randomized between receiving BioGlue or Peri-strips as an adjunct to the stapling line (15). Comparing the two arms of the study, the duration of air leak was 3±4.6 days (mean ± SD) in the BioGlue arm compared with 6.5±6.88 days in the Peri-strips arm (P=0.27), intercostal drainage was 733±404 versus 1,001±861 (P=0.65) and duration of intercostal drainage was 9.7±10.6 versus 11.5±11.1 days (P=0.73) in the two groups, respectively.
Tansley et al. compared two groups of patients; one was treated only surgically and the other was treated with BioGlue in addition to the standard surgical procedure (9). Patients from the latter group had shorter duration of air leak, intercostal drainage, and LOS, as shown in Table 2.
Finally, Potaris et al. applied BioGlue in 21 patients who underwent bullectomy and compared the results with an age- and sex-matched control group of similar patients (16). Duration of air leak was significantly shorter in the BioGlue group, as well as duration of intercostal drainage. The length of stay (LOS) was reduced but this did not reach statistically significant figure compared with the control group.
All complications in the BioGlue-treated groups of each study were not major and are outlined in Table 2 as well.
Management of BPF
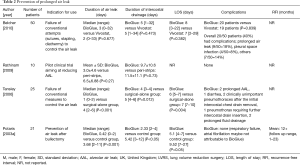
Six studies were included in this section (12,13,18-21) and their features are summarized in Table 3. In all cases, BPF treatment required secondary intervention. Amongst them, three studies refer to the treatment of BPF using an Amplatzer vascular occlusion device in combination with BioGlue application (12,13,21). Complications following this strategy were minor with two studies referring to re-application of BioGlue three days and three weeks after the initial procedure, respectively (13,21). Patients were all well after a follow-up period of 6 and 12 months in one (12) and 14 months in another study (13).
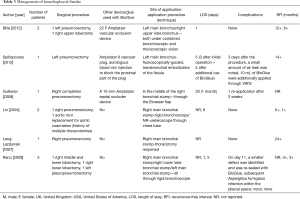
Full table
Lin et al. described the sealing of BPF with BioGlue in two patients who had undergone right pneumonectomy and aortic root replacement respectively. No complications were encountered postoperatively as well as after one and five months respectively (18).
In addition, Lang-Lazdunski described the successful closure of a small BPF after injection of BioGlue on the right main bronchial stump following pneumonectomy. No recurrence was recorded at two years follow-up (19).
Ranu et al. reported the application of BioGlue in three patients who had developed BPF after major thoracic operations (20). One of them developed a smaller defect on day 11, which was eventually sealed by re-application of BioGlue. At follow-up, two patients remained well, while the third, despite subsequent pleural infection, had the bronchial stump intact at repeat bronchoscopy.
Mixed studies
Two studies were found to present combined results; hence they are described in a distinct category (10,17).
In the study by Potaris et al., BioGlue was applied in 38 patients, with mean air leak duration of 0.6 days (range, 0–2 days); mean intercostal drainage of 3.4 days (range, 1–12 days) and median hospitalization of 6 days (range, 4–16 days). Amongst them, BPFs in two patients were sealed after primary operation using BioGlue, with air leak lasting 0 and 2 days. Overall, complications occurred in three patients with atelectasis in one and residual space in two (17).
Passage et al. applied BioGlue for AAL in 36 patients, BPF in 2 and lymph leak in 2 patients (10). In the AAL-group, BioGlue was used in 30 patients during the primary procedure, while persistent air leak necessitated its application after primary operation in six cases. Time from initial procedure to re-intervention was 7.7 days (range, 1–21 days), hospital stay was 10 days (range, 1–78 days) and mean duration of intercostal drainage was 4 days. BioGlue controlled the air leak in all but one patient, who eventually died from respiratory failure on the 19th postoperative day. In addition, two patients required re-application of glue, one developed empyema and two developed pneumonia postoperatively. Finally, in the BPF and lymph leak groups, the application of BioGlue was performed during the primary procedure in one patient of each group and it was proved effective in the half of each group’s cases (10).
Discussion
BioGlue is a commonly used surgical sealant in thoracic surgery. Our review points out that the main indications for its application are prevention of AAL and management of BPFs. As revealed by the included studies, no superior efficacy of BioGlue was shown, compared with other adjuncts such as Vivostat (14) and Peri-strips (15). We observed though a significant reduction in the duration of air leak, intercostal drainage and LOS when compared with surgical intervention alone (9,16).
In managing BPFs, BioGlue was applied in only fourteen patients (10,12,13,17-21), of which three received an Amplatzer device as well (12,13,21). No major complications were recorded. However, due to the small sample of patients, no definite conclusions concerning its efficacy can be drawn.
Prolonged air leak is considered the most common complication following thoracic surgery operations (23). Drahush et al. proposed a standardized approach to reduce prolonged air leak after pulmonary resection, consisting of “fissure-last” surgical technique, staple line buttressing and protocol-driven chest tube management postoperatively. Their results revealed a 52% reduction in the incidence of air leak in comparison with the Society of Thoracic Surgeons National Database figures (24).
At a recent meta-analysis, the intraoperative use of surgical sealants or adjuncts reduced the incidence of prolonged air leak postoperatively (25). However, BioGlue was not included in the list of utilized adjuncts. In our review, only two studies showed statistically significant results in terms of duration of air leak, intercostal drainage and LOS, following the use of BioGlue (9,16).
Another issue that merits special consideration is the management of BPF, which usually present with a lower incidence after thoracic operations but yet have a detrimental effect on patient outcomes.
In terms of management, we reported three studies, in which BPFs were treated with an Amplatzer vascular occlusion device in combination with BioGlue (12,13,21). A recent case report described the treatment of a large BPF with the same device (Amplatzer) without applying BioGlue but with similar results (26). Fuso et al. compared two groups of patients who developed BPFs, one treated conservatively and a second undergoing conservative treatment plus endoscopic application of different glues (27). The results revealed a shorter resolution time in the combined-treated group (15.4±13.2 vs. 25.8±13.2 days, P=0.299), which though not statistically significant, was related to a larger fistula size. In general, large BPFs (>8 mm) are not considered suitable for endoscopic management, whereas smaller BPFs are more likely to heal properly (28). Unfortunately, our studies did not provide details on the size of BPF and therefore no firm conclusions can be drawn on the efficacy of BioGlue in sealing any size of BPF.
Since video-assisted thoracoscopic surgery (VATS) is becoming the dominant modality in thoracic surgery, application of BioGlue may be possible through less invasive approaches. In our review, one study referred to successful application of BioGlue in two cases during VATS, one after wedge resection and the other following an iatrogenic lung laceration (17). However, most of the studies reporting on the prevention of AAL did not provide details on the surgical procedures that were implemented. Furthermore, the vast majority of studies concerning the treatment of BPF reported the application of this adjunct through an endoscopic approach (12,13,18,20). The variability in applicator lengths renders the use of this glue feasible not only during thoracotomies but also during VATS or rigid bronchoscopy (17). Despite the limited evidence to date, no technical restrictions seem to emerge with regards to the application procedure, thus suggesting the applicability of BioGlue during minimally invasive approaches.
There remain concerns about the safety of BioGlue due to its non-human nature. In general, BioGlue comprises of two components, purified BSA and glutaraldehyde which produce a mechanical seal when bound to each other (29). This seal remains rigid and does not expand with the underlying lung parenchyma resulting in increased risk of translocation and re-establishment of air leak. Additionally it has a low bio absorbability (14,17,19), while its non-autologous nature can trigger an inflammatory response (30), with risk of toxicity (31) and lung fibrosis (32).
Conclusions
BioGlue seems to be used by the Thoracic Community for the prevention of AAL and less frequently for the management of BPF. Although small randomized controlled trials quote its efficiency in the management of AAL, its benefit in treating BPF has yet to be proven through studies with a larger cohort of patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wood DE, Lauer LM, Layton A, et al. Prolonged length of stay associated with air leak following pulmonary resection has a negative impact on hospital margin. Clinicoecon Outcomes Res 2016;8:187-95. [Crossref] [PubMed]
- Keagy BA, Lores ME, Starek PJ, et al. Elective pulmonary lobectomy: factors associated with morbidity and operative mortality. Ann Thorac Surg 1985;40:349-52. [Crossref] [PubMed]
- Duque JL, Ramos G, Castrodeza J, et al. Early complications in surgical treatment of lung cancer: a prospective, multicenter study. Grupo Cooperativo de Carcinoma Broncogénico de la Sociedad Española de Neumología y Cirugía Torácica. Ann Thorac Surg 1997;63:944-50. [Crossref] [PubMed]
- Droghetti A, Schiavini A, Muriana P, et al. A prospective randomized trial comparing completion technique of fissures for lobectomy: stapler versus precision dissection and sealant. J Thorac Cardiovasc Surg 2008;136:383-91. [Crossref] [PubMed]
- Sirbu H, Busch T, Aleksic I, et al. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg 2001;7:330-6. [PubMed]
- Regnard JF, Alifano M, Puyo P, et al. Open window thoracostomy followed by intrathoracic flap transposition in the treatment of empyema complicating pulmonary resection. J Thorac Cardiovasc Surg 2000;120:270-5. [Crossref] [PubMed]
- Matthew TL, Spotnitz WD, Kron IL, et al. Four years' experience with fibrin sealant in thoracic and cardiovascular surgery. Ann Thorac Surg 1990;50:40-3; discussion 43-4. [Crossref] [PubMed]
- Fuller C. Reduction of intraoperative air leaks with Progel in pulmonary resection: a comprehensive review. J Cardiothorac Surg 2013;8:90. [Crossref] [PubMed]
- Tansley P, Al-Mulhim F, Lim E, et al. A prospective, randomized, controlled trial of the effectiveness of BioGlue in treating alveolar air leaks. J Thorac Cardiovasc Surg 2006;132:105-12. [Crossref] [PubMed]
- Passage J, Tam R, Windsor M, et al. Bioglue: a review of the use of this new surgical adhesive in thoracic surgery. ANZ J Surg 2005;75:315-8. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. [Crossref] [PubMed]
- Billè A, Sabarwhal T, Tom R. Vascular occlusion device closure of bronchial stump fistulae: a straightforward approach to manage bronchial stump breakdown. Gen Thorac Cardiovasc Surg 2012;60:847-50. [Crossref] [PubMed]
- Spiliopoulos S, Krokidis M, Gkoutzios P, et al. Successful exclusion of a large bronchopleural fistula using an Amplatzer II vascular plug and glue embolization. Acta Radiol 2012;53:406-9. [Crossref] [PubMed]
- Belcher E, Dusmet M, Jordan S, et al. A prospective, randomized trial comparing BioGlue and Vivostat for the control of alveolar air leak. J Thorac Cardiovasc Surg 2010;140:32-8. [Crossref] [PubMed]
- Rathinam S, Naidu BV, Nanjaiah P, et al. BioGlue and Peri-strips in lung volume reduction surgery: pilot randomised controlled trial. J Cardiothorac Surg 2009;4:37. [Crossref] [PubMed]
- Potaris K, Mihos P, Gakidis I. Experience with an albumin-glutaraldehyde tissue adhesive in sealing air leaks after bullectomy. Heart Surg Forum 2003;6:429-33. [PubMed]
- Potaris K, Mihos P, Gakidis I. Preliminary results with the use of an albumin-glutaraldehyde tissue adhesive in lung surgery. Med Sci Monit 2003;9:PI79-83. [PubMed]
- Lin J, Iannettoni MD. Closure of bronchopleural fistulas using albumin-glutaraldehyde tissue adhesive. Ann Thorac Surg 2004;77:326-8. [Crossref] [PubMed]
- Lang-Lazdunski L. Closure of a bronchopleural fistula after extended right pneumonectomy after induction chemotherapy with BioGlue surgical adhesive. J Thorac Cardiovasc Surg 2006;132:1497-8. [Crossref] [PubMed]
- Ranu H, Gatheral T, Sheth A, et al. Successful endobronchial seal of surgical bronchopleural fistulas using BioGlue. Ann Thorac Surg 2009;88:1691-2. [Crossref] [PubMed]
- Gulkarov I, Paul S, Altorki NK, et al. Use of Amplatzer device for endobronchial closure of bronchopleural fistulas. Interact Cardiovasc Thorac Surg 2009;9:901-2. [Crossref] [PubMed]
- Katoch CD, Chandran VM, Bhattacharyya D, et al. Closure of bronchopleural fistula by interventional bronchoscopy using sealants and endobronchial devices. Med J Armed Forces India 2013;69:326-9. [Crossref] [PubMed]
- Abolhoda A, Liu D, Brooks A, et al. Prolonged air leak following radical upper lobectomy: an analysis of incidence and possible risk factors. Chest 1998;113:1507-10. [Crossref] [PubMed]
- Drahush N, Miller AD, Smith JS, et al. Standardized Approach to Prolonged Air Leak Reduction After Pulmonary Resection. Ann Thorac Surg 2016;101:2097-101. [Crossref] [PubMed]
- Malapert G, Hanna HA, Pages PB, et al. Surgical sealant for the prevention of prolonged air leak after lung resection: meta-analysis. Ann Thorac Surg 2010;90:1779-85. [Crossref] [PubMed]
- Delanote I, Budts W, De Leyn P, et al. Large Bronchopleural Fistula After Surgical Resection: Secret to Success. J Thorac Oncol 2016;11:268-9. [Crossref] [PubMed]
- Fuso L, Varone F, Nachira D, et al. Incidence and Management of Post-Lobectomy and Pneumonectomy Bronchopleural Fistula. Lung 2016;194:299-305. [Crossref] [PubMed]
- Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest 2005;128:3955-65. [Crossref] [PubMed]
- Bhamidipati CM, Coselli JS, LeMaire SA. BioGlue in 2011: what is its role in cardiac surgery? J Extra Corpor Technol 2012;44:6-12. [PubMed]
- Erasmi AW, Sievers HH, Wolschläger C. Inflammatory response after BioGlue application. Ann Thorac Surg 2002;73:1025-6. [Crossref] [PubMed]
- Fürst W, Banerjee A. Release of glutaraldehyde from an albumin-glutaraldehyde tissue adhesive causes significant in vitro and in vivo toxicity. Ann Thorac Surg 2005;79:1522-8; discussion 1529. [Crossref] [PubMed]
- Haj-Yahia S, Mittal T, Birks E, et al. Lung fibrosis as a potential complication of the hemostatic tissue sealant, biologic glue (Bioglue). J Thorac Cardiovasc Surg 2007;133:1387-8. [Crossref] [PubMed]