The mechanisms of radioresistance in esophageal squamous cell carcinoma and current strategies in radiosensitivity
Introduction
Esophageal cancer is the eighth most common cancer worldwide. In 2015, approximately 18,170 people were diagnosed with esophageal cancer, and 15,450 people died of the disease in the US (1). Esophageal cancers are divided into two histological groups: squamous cell carcinoma (SCC) and adenocarcinoma (AC). SCC is common in Asia, especially in China, while AC is common in North America and in Western countries. SCC accounts for up to 90% of all esophageal cancers, but the incidence of AC has surpassed that of SCC in North America and Western countries, especially in white men compared with white women (2). Barrett’s esophagus is recognized as a precursor lesion of AC, which primarily originates in the lower third of the esophagus (3). Radiotherapy has become an important treatment modality, especially for those patients with unresectable esophageal cancer. Free radicals produced by ionizing radiation may directly affect the DNA or they may indirectly affect other cellular molecules, especially H2O. These radicals induce the formation of reactive oxygen species (ROS) and subsequent oxidative stress (4). However, resistance to radiation results in relapse and treatment failure. Modalities for the improvement of radiosensitivity are urgently needed for clinical application. The effect of radiotherapy alone is limited, but concurrent chemoradiotherapy and targeted therapy can significantly improve survival rates and control local-regional recurrence in patients with esophageal cancer.
Cellular mechanisms of radioresistance
Cell cycle checkpoint regulation
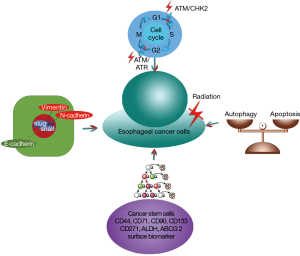
The cell cycle checkpoint signaling pathway is a critical process that protects cancer cells from DNA damage. ATM is a phosphatidylinositol kinase-related protein that modulates cell cycle checkpoints after DNA damage is induced by ionizing radiation. Activation of ATM results in dimer dissociation, autophosphorylation and the phosphorylation of downstream proteins including p53, CHK2, and RAD9, among others. Cells are often blocked in the G1/S or G2/M phases, which provides time for cells to repair DNA double-strand breaks (DSBs). Activation of the Cyclin E/CDK2 complex controls G1/S transition through the p53/p21 pathway (5). p53 is a key cell cycle checkpoint regulatory protein that induces G1/S arrest through the activation of p21, which belongs to the Cip/Kip family of CDK inhibitors. Activation of the Cdc2/Cyclin B complex controls G2/M transition through the CHK2 pathway (6). Radiation-induced G1/S arrest prevents the replication of damaged DNA and subsequent entry into S phase, while G2/M arrest prevents the segregation of aberrant chromosomes prior to entry into M phase. Apoptosis is induced to remove the damaged cells if the damage is irreversible or if the phase is dysfunctional. In short, radiation-induced cell cycle checkpoint signaling pathways protect cells from radiation damage and promote the survival of cancer cells. Numerous studies have demonstrated that abrogation of the G2 checkpoint enhances the radioresponse in esophageal cancer cells. Qin et al. revealed that the small molecule inhibitor YM155 enhances radiosensitivity through the abrogation of the G2 checkpoint and the suppression of homologous recombination repair in esophageal SCC (7). Che et al. found that the COX-2 inhibitor NS398 enhances radiosensitivity in radioresistant esophageal cancer ECA109R50Gy cells through redistribution of the cell cycle, inhibition of expression of the catalytic subunit of DNA-dependent protein kinases and induction of tumor cell apoptosis (8).
Cancer stem cells (CSCs)
Esophageal cancer stem cells (ECSCs) are populations of esophageal cancer cells that possess stem cell properties and that can promote the initiation of tumors whose cells have the ability to self-renew. CD44, CD71, CD90, CD133, CD271, aldehyde dehydrogenase (ALDH), and ATP-binding cassette subfamily G member 2 (ABCG2) have been reported as potential cell surface markers of ECSCs (9-11). The mechanisms by which ECSCs become radioresistant are as follows: (I) DNA repair. The DNA DSBs that occur following radiation are mainly repaired by nonhomologous end joining (NHEJ), which involves repair and recognition by genes such as ATM, XECC4, Ligase 4 and DNA-PKcs. Accumulating evidence has revealed that the ATM signaling pathway is more active in CSCs than in normal cancer cells. Chen et al. isolated ECSC, as a side population (SP), from normal esophageal cancer (EC9706 cells) and found that ECSCs avoided apoptosis through a decrease in DNA damage and an increase in DNA damage repair (12). Qian et al. found that human positive cofactor 4 (PC4) plays a critical role in NHEJ and DNA damage repair and that the knockdown of PC4 increases apoptosis and mitotic catastrophe (MC) induced by radiation in esophageal SCC (13); (II) cycle distribution. The radiosensitivity of esophageal cancer cells changes as they progress through the different cell cycle phases. Cells exhibit more radiosensitivity in the mitotic phase and more radioresistance in late S phase. CSCs are common in the cell cycle phase in which cells are quiescent. Following radiation, checkpoint kinases activate the ATM and ATR signaling pathways in CSCs to a greater extent than in normal esophageal cancer cells; (III) free radical and ROS scavenging. CSCs can decrease the level of ROS following radiation through the activation of ROS scavenging enzymes such as superoxide dismutase (SOD) and glutathione (GSH). GSH is an intracellular antioxidant molecule whose synthesis is catalyzed by the regulatory subunit of the glutamate-cysteine ligase. As a glutamate-cysteine ligase inhibitor, buthionine sulfoximine (BSO) decreases the colony formation ability of CSCs and increases the antioxidant ability and radiosensitivity of CSCs (14). The activation of markers of radiosensitivity, such as the transcription factors Nrf2 and nuclear factor κB (NF-κB), improves the potency of ROS scavenging enzymes. (IV) Interaction with the stromal microenvironment. Resident fibroblasts secret transforming growth factor β (TGF-β) and promote epithelial-mesenchymal transition (EMT) in CSCs, which could decrease radiosensitivity. CD44 is an extracellular matrix receptor that is expressed on the surface of CSCs and is related to the degree of malignancy.
EMT
EMT is a process through which epithelial cells acquire mesenchymal properties during embryonic development and cancer progression. EMT is characterized by the loss of the epithelial marker E-cadherin and the acquisition of mesenchymal markers including N-cadherin and Vimentin, among others. E-cadherin is a cell adhesion molecule that plays a critical role in the maintenance of epithelial structure. Repression of E-cadherin is the key step in EMT, and this progression may be modulated by the zinc finger proteins Snail and Slug. EMT has been reported to be associated with poor prognosis and chemoradioresistance in numerous malignances. In addition, irradiation might promote the migration and invasiveness of esophageal cancer cells through the EMT process. He et al. developed a radioresistant esophageal cancer cell line (KYSE-150RR) via fractional radiation and found that radiation-induced EMT occurred primarily through the PTEN-dependent Akt/Snail signaling pathway (15).
Multiple pro-survival and pro-proliferation signaling pathways
Aberrant Wnt/β-catenin signaling can lead to chromosomal instability and tolerance of DNA damage through its regulation of the mitotic spindle (16). The Wnt signaling pathway downregulates the level of the antiapoptotic gene Bcl-2 as well as the levels of phospho-Akt and upregulates the proapoptotic gene Caspase-3 to drive normal stem cells to become CSCs. Epidermal growth factor receptor (EGFR) and G-protein-coupled receptors activate the PI3K-Akt-mTOR signaling pathway to promote tumor cell growth, proliferation and survival via the inhibition of apoptosis (17). JAK or Src tyrosine kinase activates STAT3, which functions in tumor cell proliferation, differentiation and survival. Autophagy, as a conserved process, mediates the degradation of dysfunctional organelles and the turnover of long-lived proteins and limits the effect of radiotherapy through its support of metabolic mechanisms in conditions of cellular stress (18). Su et al. revealed that FH535 increases the radiosensitivity of radioresistant esophageal cancer KYSE-150 cells (KYSE-150R) through a reversal of the expression of Wnt/beta-catenin signaling pathway proteins (Wnt 1, FZD1-4, GSK3β, CTNNB1 and Cyclin D1) (19) (Figure 1).
Tumor-associated microenvironment (TAM) and radioresistance
Hypoxia and the HIF-1 pathway
Hypoxia, as a primary mechanism of resistance to radiotherapy and a pathophysiologic characteristic of malignant tumors, interferes with the repair of DNA damage (20). Cancer cell hypoxia often results from the fast rate of tumor growth and when tumors require more than the limited distribution of oxygen within blood vessels (21). At the same time, abnormal angiogenesis and poor vascular function also result in reduced oxygen tension (22). Accumulating evidence from radiation biology and oncology studies has revealed that cancer cells under hypoxic conditions are approximately 2–3 times more radioresistant than those under normal conditions. Radiosensitivity is slowly reduced when the pressure of O2 is less than 30 mmHg, while cells are maximally radioresistant when the pressure is less than 0.5 mmHg (23). HIF-1, an important transcription factor, induces the expression of multiple genes associated with cellular metabolism, metastasis of tumor cells and angiogenesis. HIF-1, as a heterodimeric factor, contains an α-subunit (HIF-1α) and a β-subunit (HIF-1β). Under normal oxygen conditions, HIF-1α is rapidly degraded through hydroxylation by prolyl hydroxylases (PHDs) and is ubiquitinated by a pVHL-containing E3 ubiquitin ligase. However, under hypoxic conditions, HIF-1α remains stable (24). Using optical imaging, Harada et al. revealed that ionizing radiation can activate HIF-1α through a HIF-1α-dependent gene. In esophageal cancer, radiation upregulates the expression of HIF-1α through an improvement in oxidative stress and an increase in the availabilities of glucose and oxygen. Subsequently, HIF-1α increases the expression of VEGF, which protects vascular endothelial cells against the cytotoxic effects of radiation (25). Yang et al. demonstrated that berberine enhanced the radiosensitivity of esophageal cancer via the inhibition of VEGF and HIF-1α in vitro and in vivo (26). Zhu et al. found that recombinant human endostatin could enhance the radiosensitivity of esophageal SCC via the downregulation of the expression of VEGF and HIF-1α after radiation therapy and via normalization of the tumor vasculature (27).
Cancer-associated fibroblasts (CAFs)
CAFs have been reported to be abundant in the stroma in many cancer types and are regarded to play a critical role in the development and progression of esophageal cancer and in the promotion of cancer proliferation, invasion, metastasis, and angiogenesis (28). CAFs originate from cells with an activated myofibroblast-like phenotype and are recognized by high levels of fibroblast activation protein-α (FAP) and α-smooth muscle actin (α-SMA). Underwood et al. found that most patients with esophageal adenocarcinoma (EAC) express high levels of stromal α-SMA, which predicts a poor survival rate and a poor prognosis. They also observed that α-SMA may increase the invasion potential of esophageal cancer cells through the disruption of the periostin and PI3K-AKT signaling pathways (28). Ji et al. indicated that CAFs decrease the radiosensitivity of the lung cancer cell lines A549 and H1299, which significantly contributes to the proliferation and survival of these cancer cells (29).
Tumor-associated macrophages
Tumors have a complex microenvironment that maintains the malignant potential of the tumor and promotes cancer cell invasion and migration; the most abundant cells in the microenvironment are macrophages (30). Macrophages can be divided into two subpopulations of cells. The M1 subpopulation is activated by Toll-like receptor ligands and interferon-γ and plays a role in antitumor immunity, while the M2 subpopulation is activated by interleukin 4 (IL-4) or interleukin 13 (IL-13), each of which suppresses antitumor immunity. Myeloid-derived suppressor cells (MDSCs) are precursors of tumor-associated macrophages and dendritic cells (DCs). Tumor-infiltrating macrophages, which have a predominantly polarized M2 phenotype, play a significant role in the disruption of adaptive immunity; they also contribute to the processes of tumor development and progression (31). M1 macrophages express high levels of major histocompatibility complex class II in normoxic tumor tissues and antiangiogenic chemokines such as CXCL9 and CXCL10. M2 macrophages express low levels of major histocompatibility complex class II in hypoxic tumor tissues and proangiogenic chemokines such as CCL17, CCL22, and CCL24. Tumor-associated macrophages secrete a large number of growth factors such as PDGF, FGF family members, VEGF, and TGF-β, which play critical proangiogenic roles in esophageal SCC (32). Tumor-associated macrophages also release proteases and matrix proteins such as MMPs, cathepsins and serine proteases to regulate the composition of the ECM and to increase disruption of the basement membrane. Several anti-macrophage approaches, such as the use of a CCL5 receptor antagonist (Met-CCL5), have been evaluated recently; this treatment could downregulate the numbers of tumor-infiltrating macrophages and significantly decreased the tumor volume after radiotherapy in a murine model of esophageal cancer (33). Zoledronic acid and liposomal clodronate reduce the invasion and metastasis of irradiated esophageal SCC through the depletion of tumor-infiltrating CD11b+ monocytes/macrophages that express MMP9.
Regulatory T cells (Tregs)
CD4+CD25+ Tregs account for approximately 5% of T cells and are recognizable by the expression of FoxP3, which is a transcription factor that is essential for cancer development and progression. Through their suppressive function, Tregs play a critical role in protecting the body against autoimmunity and tissue damage (34,35). Tregs may either be natural Tregs (nTregs) or inducible Tregs (iTregs). nTregs originate in the thymus and mediate suppressive functions through the perforin/granzyme or Fas/Fasl pathways, while inducible Tregs (iTregs) are induced outside the thymus after they are exposed to IL-2, TGF-β and IL-10. Tregs negatively regulate T cell immune responses in vivo and promote the invasion, proliferation and metastasis of esophageal cancer (36). Radiotherapy could lead to the formation of a chronic inflammatory microenvironment through modulation of the host immune system, which increases the frequency of Tregs and results in radioresistance and recurrence of malignant tumors. Daclizumab (an anti-CD25 Ab) and the tyrosine kinase inhibitor sunitinib have been used to increase anti-tumor immunity through a reduction in the frequency of Tregs.
Other stromal cells and molecules
Dendritic cells, as potent antigen presenting cells (APCs), present antigens to antigen-specific T cells and mediate the innate and adaptive immune responses (37). Dendritic cells are derived from bone marrow hematopoietic progenitor cells, but they mature within peripheral tissues. Dendritic cells play a dual role in the TAM such as in the mediation of potential anti-tumor immune responses, the activation of cytotoxic T lymphocytes (CTLs) and the blockade of anti-tumor immune responses. Exosomes are multivesicular bodies (MVBs) approximately 30-120 nm in diameter that are derived from luminal membranes; they include abundant bioactive molecules such as miRNA, mRNA, DNA, lipids and proteins (38). Exosomes participate in communication between cells and play a significant role in the balance between development and homeostasis in normal tissues and during oncogenesis. Jelonek et al. revealed that exosomes alter their proteins and miRNAs to exert a radioresistant effect (39). Boelens et al. found that exosomes derived from the coculture of stromal and breast cancer cells mediate chemoradioresistance through paracrine and juxtacrine signaling (32,40) (Figure 2).

Targeted therapy in combination with chemoradiotherapy
The ErbB family includes four tyrosine kinases: ErbB-1 (EGFR), ErbB-2 (HER 2), ErbB-3 (HER 3), and ErbB-4 (HER 4). EGFR is overexpressed in approximately 50-71% of SCC patients and in 9–55% of AC patients (41). EGFR overexpression is associated with a poor prognosis and poor overall survival and is activated by many ligands including EGF, TGF-α and epiregulin. Anti-EGFR monoclonal antibodies (cetuximab and panitumumab) and tyrosine kinase inhibitors (gefitinib and erlotinib) have achieved a significant benefit in clinical trials (32,40,42,43). Safran et al. treated 57 patients with esophageal cancer with cetuximab, paclitaxel and RT at a dose of 50.4 Gy/cfx and found that 70% of the patients had a complete clinical response after chemoradiotherapy (44). HER2 is usually identified on the cell surface by immunohistochemistry (IHC) or in the nucleus by fluorescence in situ hybridization (FISH) (45). Overexpression of HER2 is common in patients with SCC and in those with AC (approximately 23% and 22%, respectively) and is associated with a poor survival rate (46). The anti-HER2 monoclonal antibody trastuzumab has been demonstrated to improve the survival rate of patients with metastatic HER2-positive esophageal cancer, but the effect of the HER2 tyrosine kinase inhibitor lapatinib is still controversial. In a Phase III trial of 584 HER2-positive esophageal cancer patients, trastuzumab was given along with paclitaxel and radiation at a dose of 50.4 Gy/cfx. The median overall survival time was 14.8 vs. 11.1 months in the trastuzumab + chemoradiotherapy group and the chemoradiotherapy group, respectively.
VEGF is a critical regulator of both physiologic and pathologic angiogenesis. VEGF can induce endothelial cell mitogenesis, invasion and vascular permeability, and can mediate tumor growth and metastasis. Overexpression of VEGF is associated with a poor survival rate and advanced cancer stage in 30–60% patients with esophageal cancer; VEGF overexpression also contributes to tumor recurrence and metastasis (47). VEGFR is a predictor of poor prognosis and is overexpressed in 30–50% of esophageal SCC cases. In a retrospective study of 117 esophageal cancer patients conducted by Shih et al., it was demonstrated that the mean number of metastatic lymph nodes was 5.6 vs. 3.0 in VEGF-positive cases and VEGF-negative cases, respectively (48). The anti-VEGF monoclonal antibody bevacizumab and the VEGFR tyrosine kinase inhibitor sorafenib have been reported to increase the efficacy of chemoradiotherapy in esophageal cancer patients (49). Meluch et al. added bevacizumab, erlotinib, carboplatin and paclitaxel to RT at a dose of 45 Gy (cfx) and treated 62 patients with locally advanced esophageal cancer; the pCR rate was 30% (50).
c-Met is a transmembrane receptor tyrosine kinase, and hepatocyte growth factor (HGF) is the only ligand that binds to this receptor. Aberrant activation of the HGF/Met signaling pathway has been demonstrated to promote the progression and metastasis of esophageal cancer (51). Overexpression of c-Met is associated with an aggressive phenotype and a poor prognosis in patients with esophageal cancer. c-Met promotes motility, proliferation, metastasis and angiogenesis in esophageal SCC through the RAS-MAPK and PI3K-Akt signaling pathways (52). c-Met inhibitors (tivantinib, crizotinib, foretinib) and an HGF inhibitor (rilotumumab) were reported to increase the efficacy of chemoradiotherapy in multiple clinical trials.
Gene expression profiling
Gene expression microarray is a novel high-throughput technology that has been widely used for the identification of the biological characteristics of malignant tumors such as esophageal cancer. Gene expression profile microarrays can analyze thousands of genes and can identify the relevant genes that are related to tumor prognosis (53). In particular, gene expression microarrays have achieved a benefit in terms of their ability to predict responses to neoadjuvant chemoradiotherapy. Maher et al. found that five biomarkers (EPB41L3, RTKN, STAT5B, NMES1 and RNPC1) could improve the accuracy in the prediction of the radioresponse of 13 patients with esophageal cancer through DNA microarrays, which were then validated by RT-PCR (54). Duong et al. analyzed a group of 46 esophageal cancer patients, which consisted of 21 SCC and 25 AC patients who received neoadjuvant CRT, and found that 32 genes could be used to predict radioresponse by DNA microarray (55). Guo et al. revealed that aberrant hypermethylation of RASSF2 is associated with a poor prognosis and that peripheral blood DNA could be used to predict the radioresponse of patients with esophageal cancer (56).
Single nucleotide polymorphisms (SNPs)
As the sequence of the human genome was revealed, we found that genetic variation is larger than previously thought and that the most common variations are SNPs. SNPs have been used to analyze cancer treatment outcome predictor (CTOP) genes and to judge therapeutic effects in esophageal cancer, as most SNPs are silent (57). Nucleotide excision repair genes such as ERCC1 and XRCC1 protect the genome against multiple DNA lesions caused by ionizing radiation. Wu et al. investigated variations in SNPs in 210 patients with esophageal cancer using pathway-based approaches and found that the variant allele R399Q in the XRCC1 gene is related to a poor response and could be a prognostic marker in clinical patients (58). Yu et al. found that the C118T SNP in the ERCC1 gene could predict response to neoadjuvant radiochemotherapy in 52 patients with esophageal SCC (59).
MicroRNAs
miRNAs are short noncoding RNA sequences 19–24 nucleotides in length that can regulate gene expression through the inhibition of mRNA translation. It has been confirmed that miRNAs are present in tissues and body fluids, where they play a critical role in the progression and recurrence of cancers (60). Odenthal et al. analyzed 768 miRNAs using pretherapeutic and post-therapeutic biopsies of 80 esophageal cancer patients and found that miR-192 and miR-194 are significantly related to histopathologic response after neoadjuvant chemoradiotherapy (61). Zhou et al. compared miRNA expression in primary ESCCs and recurrent ESCCs after radiotherapy and found that overexpression of miRNA-381 is significantly associated with a decrease in tumor growth and an increase in the radiosensitivity of esophageal cell carcinoma patients (62). Li et al. studied 38 patients with ESCC and 19 healthy individuals and found that high levels of plasma miRNA-16 and miRNA-21 are associated with a decrease in progression-free survival (P=0.031 and P=0.038 for miRNA-16 and miRNA-21, respectively) (63).
Proteomics
Proteomics involves the determination of the function of genomic translation and the tumor phenotypes that regulate cancer behavior (64). Proteins are superior biomarkers than other molecules because they influence molecular pathways that are relevant to tumor progression and metastasis (65). Maher et al. studied 31 patients with esophageal cancer, 16 of whom exhibited a poor response and 15 of whom exhibited a good response according to the Mandard tumor regression grade (TRG) classification system. They also observed that the serum complement factors C4a and C3a are higher in patients with a poor response and that they predict the response to chemoradiotherapy with sensitivities of 78.6% and 83.3%, respectively (66).
IHC
IHC has an advantage in providing detailed morphological information in a large number of formalin-fixed paraffin-embedded tissue samples and is used widely in the discovery of hypothesis-driven biomarkers. Smit et al. investigated esophageal cancer cells that contain a CD44+/CD24− subpopulation, which exhibit higher sphere-forming potential and a higher proliferation rate than the CD44+/CD24+ subpopulation. In a study of preneoadjuvant chemoradiotherapy, in which biopsy material from 27 esophageal cancer patients was examined, the CD44+/CD24 population was identified in 50% of patients with a poor response to chemoradiotherapy. In contrast, this subpopulation was not found in any of the patients who exhibited a complete response, which indicates that the CD44+/CD24− population can be a predictive biomarker in esophageal cancer patients in terms of their response to chemoradiotherapy (67).
Medical imaging
Imaging technologies have developed rapidly in recent years. Metabolic and functional imaging modalities such as FDG PET, functional MRI and Hypoxia PET have been used to evaluate the therapeutic effects of radiochemotherapy in patients with esophageal cancer. In their study of 31 patients with esophageal cancer, Klaassen et al. found that the hypoxia tracer (18F) HX4 demonstrated good repeatability and may be a potential way to measure treatment response (68). van Rossum et al. demonstrated that changes in the apparent diffusion coefficient (ADC) could predict pathologic response to radiotherapy in 20 patients with esophageal cancer through diffusion-weighted magnetic resonance (69).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [PubMed]
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499-509. [Crossref] [PubMed]
- Runge TM, Abrams JA, Shaheen NJ. Epidemiology of Barrett's Esophagus and Esophageal Adenocarcinoma. Gastroenterol Clin North Am 2015;44:203-31. [Crossref] [PubMed]
- He H, Tian D, Guo J, et al. DNA damage response in peritumoral regions of oesophageal cancer microenvironment. Carcinogenesis 2013;34:139-45. [Crossref] [PubMed]
- Miyata H, Doki Y, Shiozaki H, et al. CDC25B and p53 are independently implicated in radiation sensitivity for human esophageal cancers. Clin Cancer Res 2000;6:4859-65. [PubMed]
- Miyata H, Doki Y, Yamamoto H, et al. Overexpression of CDC25B overrides radiation-induced G2-M arrest and results in increased apoptosis in esophageal cancer cells. Cancer Res 2001;61:3188-93. [PubMed]
- Qin Q, Cheng H, Lu J, et al. Small-molecule survivin inhibitor YM155 enhances radiosensitization in esophageal squamous cell carcinoma by the abrogation of G2 checkpoint and suppression of homologous recombination repair. J Hematol Oncol 2014;7:62. [Crossref] [PubMed]
- Che SM, Zhang XZ, Liu XL, et al. The radiosensitization effect of NS398 on esophageal cancer stem cell-like radioresistant cells. Dis Esophagus 2011;24:265-73. [Crossref] [PubMed]
- Nguyen GH, Murph MM, Chang JY. Cancer stem cell radioresistance and enrichment: where frontline radiation therapy may fail in lung and esophageal cancers. Cancers (Basel) 2011;3:1232-52. [Crossref] [PubMed]
- Croagh D, Frede J, Jones PH, et al. Esophageal stem cells and genetics/epigenetics in esophageal cancer. Ann N Y Acad Sci 2014;1325:8-14. [Crossref] [PubMed]
- Moncharmont C, Levy A, Gilormini M, et al. Targeting a cornerstone of radiation resistance: cancer stem cell. Cancer Lett 2012;322:139-47. [Crossref] [PubMed]
- Chen Y, Li D, Wang D, et al. Quiescence and attenuated DNA damage response promote survival of esophageal cancer stem cells. J Cell Biochem 2012;113:3643-52. [Crossref] [PubMed]
- Qian D, Zhang B, Zeng XL, et al. Inhibition of human positive cofactor 4 radiosensitizes human esophageal squmaous cell carcinoma cells by suppressing XLF-mediated nonhomologous end joining. Cell Death Dis 2014;5:e1461. [Crossref] [PubMed]
- Rycaj K, Tang DG. Cancer stem cells and radioresistance. Int J Radiat Biol 2014;90:615-21. [Crossref] [PubMed]
- He E, Pan F, Li G, et al. Fractionated Ionizing Radiation Promotes Epithelial-Mesenchymal Transition in Human Esophageal Cancer Cells through PTEN Deficiency-Mediated Akt Activation. Plos One 2015;10:e126149.
- Hein AL, Ouellette MM, Yan Y. Radiation-induced signaling pathways that promote cancer cell survival Int J Oncol 2014;45:1813-9. (review). [PubMed]
- Kuonen F, Secondini C, Ruegg C. Molecular Pathways: Emerging Pathways Mediating Growth, Invasion, and Metastasis of Tumors Progressing in an Irradiated Microenvironment. Clin Cancer Res 2012;18:5196-202. [Crossref] [PubMed]
- Nam HY, Han MW, Chang HW, et al. Prolonged autophagy by MTOR inhibitor leads radioresistant cancer cells into senescence. Autophagy 2013;9:1631-2. [Crossref] [PubMed]
- Su H, Jin X, Zhang X, et al. FH535 increases the radiosensitivity and reverses epithelial-to-mesenchymal transition of radioresistant esophageal cancer cell line KYSE-150R. J Transl Med 2015;13:104. [Crossref] [PubMed]
- Meijer TW, Kaanders JH, Span PN, et al. Targeting Hypoxia, HIF-1, and Tumor Glucose Metabolism to Improve Radiotherapy Efficacy. Clin Cancer Res 2012;18:5585-94. [Crossref] [PubMed]
- Yeom CJ, Goto Y, Zhu Y, et al. Microenvironments and Cellular Characteristics in the Micro Tumor Cords of Malignant Solid Tumors. Int J Mol Sci 2012;13:13949-65. [Crossref] [PubMed]
- Yoshimura M, Itasaka S, Harada H, et al. Microenvironment and Radiation Therapy. Biomed Res Int 2013;2013:1-13.
- Matsuo M, Matsumoto S, Mitchell JB, et al. Magnetic resonance imaging of the tumor microenvironment in radiotherapy: perfusion, hypoxia, and metabolism. Semin Radiat Oncol 2014;24:210-7. [Crossref] [PubMed]
- Kato Y, Yashiro M, Fuyuhiro Y, et al. Effects of acute and chronic hypoxia on the radiosensitivity of gastric and esophageal cancer cells. Anticancer Res 2011;31:3369-75. [PubMed]
- Yaromina A, Thames H, Zhou X, et al. Radiobiological hypoxia, histological parameters of tumour microenvironment and local tumour control after fractionated irradiation. Radiother Oncol 2010;96:116-22. [Crossref] [PubMed]
- Yang X, Yang B, Cai J, et al. Berberine enhances radiosensitivity of esophageal squamous cancer by targeting HIF-1alpha in vitro and in vivo. Cancer Biol Ther 2013;14:1068-73. [Crossref] [PubMed]
- Zhu H, Yang X, Ding Y, et al. Recombinant human endostatin enhances the radioresponse in esophageal squamous cell carcinoma by normalizing tumor vasculature and reducing hypoxia. Sci Rep 2015;5:14503. [Crossref] [PubMed]
- Underwood TJ, Hayden AL, Derouet M, et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J Pathol 2015;235:466-77. [Crossref] [PubMed]
- Ji X, Ji J, Shan F, et al. Cancer-associated fibroblasts from NSCLC promote the radioresistance in lung cancer cell lines. Int J Clin Exp Med 2015;8:7002-8. [PubMed]
- Morganti JM, Jopson TD, Liu S, et al. Cranial irradiation alters the brain's microenvironment and permits CCR2+ macrophage infiltration. PLoS One 2014;9:e93650. [Crossref] [PubMed]
- Qian BZ, Pollard JW. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010;141:39-51. [Crossref] [PubMed]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010;11:889-96. [Crossref] [PubMed]
- Shigeoka M, Urakawa N, Nakamura T, et al. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci 2013;104:1112-9. [Crossref] [PubMed]
- Izawa S, Mimura K, Watanabe M, et al. Increased prevalence of tumor-infiltrating regulatory T cells is closely related to their lower sensitivity to H2O2-induced apoptosis in gastric and esophageal cancer. Cancer Immunol Immunother 2013;62:161-70. [Crossref] [PubMed]
- Shahabi V, Postow MA, Tuck D, et al. Immune-priming of the Tumor Microenvironment by Radiotherapy. Am J Clin Oncol 2015;38:90-7. [Crossref] [PubMed]
- Tsuchikawa T, Md MM, Yamamura Y, et al. The immunological impact of neoadjuvant chemotherapy on the tumor microenvironment of esophageal squamous cell carcinoma. Ann Surg Oncol 2012;19:1713-9. [Crossref] [PubMed]
- Somja J, Demoulin S, Roncarati P, et al. Dendritic Cells in Barrett's Esophagus Carcinogenesis. Am J Pathol 2013;182:2168-79. [Crossref] [PubMed]
- Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev 2013;32:623-42. [Crossref] [PubMed]
- Jelonek K, Wojakowska A, Marczak L, et al. Ionizing radiation affects protein composition of exosomes secreted in vitro from head and neck squamous cell carcinoma. Acta Biochim Pol 2015;62:265-72. [Crossref] [PubMed]
- Boelens MC, Wu TJ, Nabet BY, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 2014;159:499-513. [Crossref] [PubMed]
- Mohamed A, El-Rayes B, Khuri FR, et al. Targeted therapies in metastatic esophageal cancer: Advances over the past decade. Crit Rev Oncol Hematol 2014;91:186-96. [Crossref] [PubMed]
- Cellini F, Valentini V. Targeted therapies in combination with radiotherapy in oesophageal and gastroesophageal carcinoma. Curr Med Chem 2014;21:990-1004. [Crossref] [PubMed]
- Ayyappan S, Prabhakar D, Sharma N. Epidermal growth factor receptor (EGFR)-targeted therapies in esophagogastric cancer. Anticancer Res 2013;33:4139-55. [PubMed]
- Safran H, Suntharalingam M, Dipetrillo T, et al. Cetuximab with concurrent chemoradiation for esophagogastric cancer: assessment of toxicity. Int J Radiat Oncol Biol Phys 2008;70:391-5. [Crossref] [PubMed]
- Kasper S, Schuler M. Targeted therapies in gastroesophageal cancer. Eur J Cancer 2014;50:1247-58. [Crossref] [PubMed]
- Won E, Janjigian YJ, Ilson DH. HER2 Directed Therapy for Gastric/Esophageal Cancers. Curr Treat Option Oncol 2014;15:395-404. [Crossref] [PubMed]
- Kordes S, Cats A, Meijer SL, et al. Targeted therapy for advanced esophagogastric adenocarcinoma. Crit Rev Oncol Hematol 2014;90:68-76. [Crossref] [PubMed]
- Shih CH, Ozawa S, Ando N, et al. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res 2000;6:1161-8. [PubMed]
- Ding M, Zhang E, He R, et al. Newly developed strategies for improving sensitivity to radiation by targeting signal pathways in cancer therapy. Cancer Sci 2013;104:1401-10. [Crossref] [PubMed]
- Meluch AA, Hainsworth JD, Gray JR, et al. Preoperative combined modality therapy with paclitaxel, carboplatin, prolonged infusion 5-fluorouracil, and radiation therapy in localized esophageal cancer: preliminary results of a Minnie Pearl Cancer Research Network phase II trial. Cancer J Sci Am 1999;5:84-91. [PubMed]
- Nantajit D, Lin D, Li JJ. The network of epithelial–mesenchymal transition: potential new targets for tumor resistance. J Cancer Res Clin Oncol 2015;141:1697-713. [Crossref] [PubMed]
- Hack SP, Bruey JM, Koeppen H. HGF/MET-directed therapeutics in gastroesophageal cancer: a review of clinical and biomarker development. Oncotarget 2014;5:2866-80. [Crossref] [PubMed]
- Schink JC, Trosman JR, Weldon CB, et al. Biomarker testing for breast, lung, and gastroesophageal cancers at NCI designated cancer centers. J Natl Cancer Inst 2014.106. [PubMed]
- Maher SG, Gillham CM, Duggan SP, et al. Gene expression analysis of diagnostic biopsies predicts pathological response to neoadjuvant chemoradiotherapy of esophageal cancer. Ann Surg 2009;250:729-37. [Crossref] [PubMed]
- Duong C, Greenawalt DM, Kowalczyk A, et al. Pretreatment gene expression profiles can be used to predict response to neoadjuvant chemoradiotherapy in esophageal cancer. Ann Surg Oncol 2007;14:3602-9. [Crossref] [PubMed]
- Guo W, Dong Z, Cui J, et al. Aberrant hypermethylation of RASSF2 in tumors and peripheral blood DNA as a biomarker for malignant progression and poor prognosis of esophageal squamous cell carcinoma. Clin Exp Metastasis 2016;33:73-85. [Crossref] [PubMed]
- Greve B, Bolling T, Amler S, et al. Evaluation of different biomarkers to predict individual radiosensitivity in an inter-laboratory comparison--lessons for future studies. PLoS One 2012;7:e47185. [Crossref] [PubMed]
- Goossens N, Nakagawa S, Sun X, et al. Cancer biomarker discovery and validation. Transl Cancer Res 2015;4:256-69. [PubMed]
- Yu X, Xiao H, Zhao B, et al. DNA repair gene ERCC1 C118T polymorphism predicts sensitivity of recurrent esophageal cancer to radiochemotherapy in a Chinese population. Thorac Cancer 2015;6:741-8. [Crossref] [PubMed]
- Gao Y, Zhu J, Zhang X, et al. BRCA1 mRNA expression as a predictive and prognostic marker in advanced esophageal squamous cell carcinoma treated with cisplatin- or docetaxel-based chemotherapy/chemoradiotherapy. PLoS One 2013;8:e52589. [Crossref] [PubMed]
- Odenthal M, Bollschweiler E, Grimminger PP, et al. MicroRNA profiling in locally advanced esophageal cancer indicates a high potential of miR-192 in prediction of multimodality therapy response. Int J Cancer 2013;133:2454-63. [Crossref] [PubMed]
- Zhou S, Ye W, Ren J, et al. MicroRNA-381 increases radiosensitivity in esophageal squamous cell carcinoma. Am J Cancer Res 2014;5:267-77. [PubMed]
- Li BX, Yu Q, Shi ZL, et al. Circulating microRNAs in esophageal squamous cell carcinoma: association with locoregional staging and survival. Int J Clin Exp Med 2015;8:7241-50. [PubMed]
- Dubeau L. Pathogenesis of serous, extra-uterine Mullerian epithelial cancer and therapeutic implications. Transl Cancer Res 2015;4:3-13. [PubMed]
- Uemura N, Kondo T. Current status of predictive biomarkers for neoadjuvant therapy in esophageal cancer. World J Gastrointest Pathophysiol 2014;5:322-34. [PubMed]
- Maher SG, McDowell DT, Collins BC, et al. Serum proteomic profiling reveals that pretreatment complement protein levels are predictive of esophageal cancer patient response to neoadjuvant chemoradiation. Ann Surg 2011;254:809-16; discussion 816-7. [Crossref] [PubMed]
- Smit JK, Faber H, Niemantsverdriet M, et al. Prediction of response to radiotherapy in the treatment of esophageal cancer using stem cell markers. Radiother Oncol 2013;107:434-41. [Crossref] [PubMed]
- Klaassen R, Bennink RJ, van Tienhoven G, et al. Feasibility and repeatability of PET with the hypoxia tracer [(18)F]HX4 in oesophageal and pancreatic cancer. Radiother Oncol 2015;116:94-9. [Crossref] [PubMed]
- van Rossum PS, van Lier AL, van Vulpen M, et al. Diffusion-weighted magnetic resonance imaging for the prediction of pathologic response to neoadjuvant chemoradiotherapy in esophageal cancer. Radiother Oncol 2015;115:163-70. [Crossref] [PubMed]


