Clinical presentation and characteristics of 25 adult cases of pulmonary sequestration
Introduction
Pulmonary (bronchopulmonary) sequestration (PS) is a nonfunctioning lung tissue, with no direct connection to normal tracheobronchial tree, PS has an independent systemic arterial vessel supplying it and the blood outflow is maintained by pulmonary or systemic veins (1). Two forms of PS based on their pleural covering are described: intralobar sequestration (ILS) and extralobar sequestration (ELS). PS is believed to be a rare congenital disease in the spectrum of the bronchopulmonary foregut malformation, with an incidence of 0.1%; true developmental nature of ILS is still controversial though (1-3).
Despite an increase in number of diagnosed cases due to the widespread of computed tomography (CT) and magnetic resonance imaging (MRI), PS is still an uncommon finding in daily clinical practice. Up to date only few series of clinical data have been published, even fewer focusing on adult population. Thus the aim of the study was to analyze clinical presentation, maintenance and short-term outcome of PS cases from a one-center perspective.
Methods
Over 110,000 hospital stays in the National Tuberculosis and Lung Diseases Research Institute, Warsaw, Poland, between January 1st, 2005 and December 31st, 2015, were taken under consideration. Using MSD engine (CompuGroup Medical Polska) we searched the Institute’s medical database for records containing any mention of PS. In the study we only included patients who have undergone surgical treatment and histological verification. Clinical information was retrospectively gathered and analyzed using Statistica v.12 (StatSoft, Inc.), we used U-Mann Whitney test and chi2 test. Presented values were rounded to two decimal points. According to our Institutional Regulations, the Ethics Committee consent was not needed.
Results
We found 25 patients meeting the criteria, 18 females and 7 males. At the time of surgery patients were between 15 and 67 years of age, the average was 38.24 (SD =14.93). The incidence of PS in our in-hospital population ranged 0.2–0.3‰.
In 7 cases patients presented with no symptoms at all—incidental radiological findings led to the surgery, the average age at the time of the procedure in this group was 34. In the symptomatic group 12 patients had infectious history (including 3 cases of lung abscess and pleural empyema), 4 presented with hemoptysis, 2 with chest pain. The infectious symptoms were prolonged and recurrent, with poor reaction to antibiotic treatment, sometimes mimicking asthma. In two cases, symptoms occurred or aggravated during pregnancy, including one spectacular case of pleural empyema in the 3rd trimester. In the symptomatic group the symptoms preceded the surgery for 2.45-year, and the average age for the surgery was 39.89. In 17 cases preoperative diagnosis with detailed characteristics of the vascularization of malfunctioning lung tissue was made using CT angiography (Figures 1,2), in one case MRI was used. In two cases radiological diagnosis indicated fibrous tumor of the pleura and bronchogenic cyst, but turned out to be wrong, respectively. Remaining cases were diagnosed during or post-surgery. On the top of 25 presented cases, in the next three, incorrect clinical and radiological diagnoses of PS was made and surgery performed—pathological examination indicated two cases of solitary fibrous tumor of the pleura and one case of congenital cystic adenomatoid malformation.
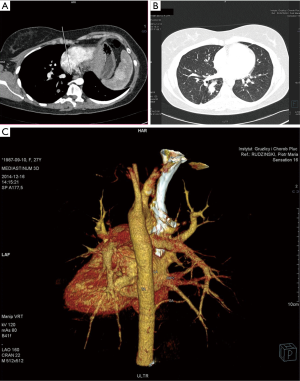
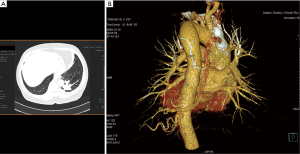
Ten patients had accompanying diseases such as breast or thyroid cancer, hypertension, sarcoidosis. Only in one case (21-year old male) congenital malformations were found: right kidney agenesis with incorrect outflow of great systemic veins and unilobar right lung (the ILS was located in the left lower lobe). Characteristics of patient are summarized in Table 1.
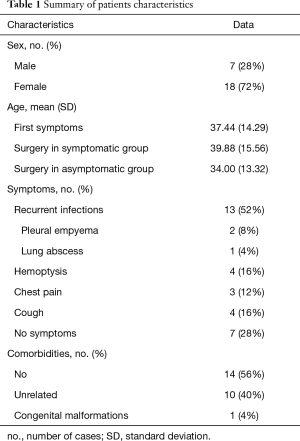
Full table
Video-assisted thoracoscopic surgery (VATS) was used in 2 cases, in another 2 cases conversion from VATS to thoracotomy was necessary due to complications. Most cases were treated with lobectomy (20/25), remaining five were treated with marginal resections. The supplying arteries were ligated using single suture ligature or endovascular clamp. Symptomatic cases were at higher risk of surgical difficulties and complications than expected, comparing to asymptomatic (chi2=4.00, df=1, P=0.04). Surgical difficulties were most likely to be related to pleural adhesions and problematic dissection of the arterial supply in the pulmonary ligament, and complications were represented by serious hemorrhages and systemic infections. A 0.53-day longer post-surgical hospital stay (mean 6.13 vs. 5.6 days) was observed in the symptomatic group, but no statistical significance was found (U=61.00, P=0.45).
Great majority of sequestrations was located in lower lobes (96%), 52% on the left. There were 22 cases of ILS, remaining 3 cases were histologically diagnosed as ELS. Pathological pattern in cases of ILS was consistent— multiple cystic areas, some air filled and some with fluid or purulent material, signs of fibrosis, chronic inflammation and vascular sclerosis (Figure 3A,B). In ELS pathological examination revealed great dilatation of bronchial ducts, filled with fluid and pathological sclerotic vasculature.
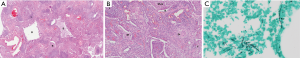
In most cases there were surgical and histological signs of infection. In 5/9 cases Aspergillus fumigatus was determined as etiological factor (Figure 3C), there were two cases of proven bacterial infection and two non-tuberculosis mycobacterial disease. Despite numerous cultures none of the patients was proven to be infected with Mycobacterium tuberculosis.
Discussion
The incidence of PS in adult population has not been established up to date, it is reported that PS stand for 0.15–6.4% of all of pulmonary malformations and most of them are diagnosed and treated during childhood (2,3); we estimated the occurrence for less than 3 per 10,000 adults. In adult patients ELS represents 6.45–7.2% of cases (3,4), less in comparison to our series—12% of cases.
The very uncommon finding would be bilateral ILS—regarded as non-existing for many decades, it was reported and discussed by Stern et al. (5); we, in our series, did not find any bilateral case.
The symptoms of PS are consistent throughout numerous data series and case reports, and similar to our findings (3,4,6-8), they are well known to most clinicians, even though being not specific. The number of asymptomatic cases was reported 9.7–15% (3), 28% in our series.
The rise of incidental diagnosis of PS should be considered—the high specificity and sensitivity of CT and MRI ease to obtain the correct diagnosis of PS (1). The differential diagnosis should focus on cystic adenomatoid malformation and bronchogenic cysts (1), also —based on our data—solitary fibrous tumor of the pleura.
The management of ELS and symptomatic cases of ILS is well established, the method of choice is surgical treatment (2,8-10). Nevertheless the cases of asymptomatic, incidentally revealed ILS are controvertial—most authors suggest an early surgery due to possible infectious complications and occult ongoing fungal infection (7,8). This standpoint is in accordance with our results showing lower rate of complications and a trend towards shorter post-surgical hospital stay. In contrast, the authors of the largest existing adult series of PS stand on the position of thoughtful qualification to preemptive surgery, mostly because there is lack of evidence when comparing surgery to observation approach (4). This ambiguity is evident also in our institution—not all of radiologically diagnosed PS cases in the last eleven years were treated with surgery.
The surgical treatment of PS for many decades was with lobectomy (rather than a lobe-sparing resection) performed by a thoracotomy. Starting from the 2000s more and more data supports safe use of VATS in the treatment of PS (3,9). This method should be only used by experienced surgeons (3) and there is an evident learning curve (9). The method was introduced in our country a few years ago so only four patients were treated with this approach; the number is increasing though—during 2014 and 2015 three out of five resections were performed using VATS. There is an ongoing discussion about the use of two-portal technique versus three- and four-portal (3,4). With the use of VATS there is better recovery and shorter hospital stay in comparison to open method (4), nevertheless conversion to open thoracotomy is sometimes needed (3), as it was in two out of four cases in our series. Some authors suggest the use of preoperative embolization of arterial supply to the sequestration to minimalize bleeding risk (11).
There are signs of infection in most cases of PS, but most of them are with no established etiological factor (4). Our own and existing data report Aspergillus sp. to be the most common infectious factor to be determined in PS patient; most of patients do not meet the criteria of invasive aspergillosis and have no indication for systemic treatment (4,7,8), the diagnosis of aspergillosis is rather based on pathological stains than microbiological cultures (4). We would suggest to take Aspergillus sp. under consideration when an empiric antimicrobial treatment is introduced to PS patient.
The current study reports data of patients who were diagnosed and treated by the same group of clinicians in a relatively short period of time, therefore the “changing-observer” error is less relevant; the number of cases is moderate when compared to two greater existing adult series of PS (6,8). The main limitation is the retrospective character and lack of statistical power to settle the most urgent questions in PS maintenance—the indications for preemptive surgery and the surgical technique of choice. Further observations to establish clear answers are needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: This work was presented on 2016 ERS International Congress London, on Sep 4, 2016.
Ethical Statement: According to our Institutional Regulations, the Ethics Committee consent was not needed.
References
- Bolca N, Topal U, Bayram S. Bronchopulmonary sequestration: radiologic findings. Eur J Radiol 2004;52:185-91. [Crossref] [PubMed]
- Corbett HJ, Humphrey GM. Pulmonary sequestration. Paediatr Respir Rev 2004;5:59-68. [Crossref] [PubMed]
- Lin CH, Chuang CY, Hsia JY, et al. Pulmonary sequestration-differences in diagnosis and treatment in a single institution. J Chin Med Assoc 2013;76:385-9. [Crossref] [PubMed]
- Sun X, Xiao Y. Pulmonary sequestration in adult patients: a retrospective study. Eur J Cardiothorac Surg 2015;48:279-82. [Crossref] [PubMed]
- Stern R, Berger S, Casaulta C, et al. Bilateral intralobar pulmonary sequestration in a newborn, case report and review of the literature on bilateral pulmonary sequestrations. J Pediatr Surg 2007;42:E19-23. [Crossref] [PubMed]
- Palmowski M, Schreiner K, Hansmann J, et al. Bronchopulmonary sequestration: a differential diagnosis in young adults for recurrent pneumonia. Lancet 2007;369:1318. [Crossref] [PubMed]
- Berna P. Pulmonary sequestration and aspergillosis. Eur J Cardiothorac Surg 2005;27:28-31. [Crossref] [PubMed]
- Halkic N, Cuénoud PF, Corthésy ME, et al. Pulmonary sequestration: a review of 26 cases. Eur J Cardiothorac Surg 1998;14:127-33. [Crossref] [PubMed]
- Kestenholz PB, Schneiter D, Hillinger S, et al. Thoracoscopic treatment of pulmonary sequestration. Eur J Cardiothorac Surg 2006;29:815-8. [Crossref] [PubMed]
- Gonzalez D, Garcia J, Fieira E, et al. Video-assisted thoracoscopic lobectomy in the treatment of intralobar pulmonary sequestration. Interact Cardiovasc Thorac Surg 2011;12:77-9. [Crossref] [PubMed]
- de Lagausie P, Bonnard A, Berrebi D, et al. Video-assisted thoracoscopic surgery for pulmonary sequestration in children. Ann Thorac Surg 2005;80:1266-9. [Crossref] [PubMed]


