Clinical analysis on 113 patients with lung cancer treated by percutaneous CT-guided microwave ablation
Introduction
Lung cancer is a kind of disease posing a serious threat to the health and life of humans, and it has a leading morbidity and mortality among various malignant tumors (1,2). Over 1.60 million people die from lung cancer each year all over the world (3). In China, the morbidity and mortality of lung cancer have always been on the rise. Due to the intensification of population aging, rural urbanization and industrialization of city and town, the environment pollution, unhealthy life-style and other factors, particularly, the increase of smoking population year by year in China, the lung cancer has a high morbidity and mortality. Currently, lung cancer has become the leading cancer in China (4).
Furthermore, in China, most patients with lung cancer have already been in middle or advanced stage when they are definitely diagnosed, and lost the opportunity of surgical resection. Some patients with early-stage lung cancer are not suitable for the surgery due to the special site of lung cancer, such as being close to the pulmonary hilus. Likewise, some patients with early-stage lung cancer accompanied by poor cardio-pulmonary function, hypertension, diabetes and other diseases gave up the surgery treatment, because these diseases make them intolerant to anesthesia and surgery. In spite of a significant improvement of lung cancer chemotherapy and radiotherapy in recent years, the overall effect is still less than satisfactory. Compared with the good survival rate brought by the radical treatment of lung cancer by surgical resection, the curative effect of chemotherapy and radiotherapy is very limited. Thus, clinically, it is urgent to provide a method which is more effective than chemotherapy and radiotherapy and nearly or even as effective as the surgical treatment to some extent for the patients who are not eligible for surgery.
Local thermal ablation therapy of tumor is the focus of domestic and overseas research in the past decade. This method is to deliver specific energy into the tumor tissue under the guidance of image technology, which make the local tissue quickly reach 60 °C where an irreversible coagulative necrosis happens, and lead to the necrosis of tumor to kill the tumor cells (5-7). Applying it to the process of treatment on lung cancer can not only kill the in situ lung cancer cells, but also protect more normal lung tissues. Thus, it requires less than the surgical treatment in terms of patient’s basic condition, thereby, it provides a viability of minimally invasive therapy for the patients with lung cancer who have a poor cardiopulmonary function and intolerance to thoracotomy.
Owing to the less trauma, easy and convenient operation, no need for general anesthesia, fast recovery after therapy and less complications, this technology conforms to the trend of the development of minimally invasive surgery, which is gradually adopted clinically, and tends to be popularized (8,9). The commonly-used thermal ablation technologies are radio frequency ablation (RFA) and microwave ablation (MWA), etc. Both RFA and MWA have been widely used for the treatment of tumours as a minimally invasive technology (5). RFA has a relatively high postoperative relapse rate, especially for the lesion sized more than 3 cm. Meanwhile, the tissues around the radio frequency needle tip is prone to be carbonized and dried, which will lead to the increase of impedance, and it will be difficult for RF heating temperature to reach the ideal ablation temperature, which is an important reason of the postoperative focal relapse (10,11). Compared with RFA, MWA has many advantages, such as high heating efficiency, fast heating, more uniformed high-temperature thermal field, thorough necrosis at the coagulative area and so on. Thus, MWA is better than RFA in terms of ablation volume, time and relapse rate (12-16). Meanwhile, MWA will not produce the electrical current which may cause the dysfunction of pacemaker, so MWA can be used for the patients with lung cancer who are installed with heart pacemakers. In view of the wide application prospect of MWA in the treatment of lung cancer, it is necessary to carry out systematic and thorough research on it. This study is to evaluate the clinical curative effect and safety of MWA on lung cancer.
Methods
Clinical data
MWA was used to treat tumor lesion of 113 patients with lung cancer who had been admitted to hospital from Jan, 2013 to Jun, 2015, the median diameter of tumor was 3.1 cm (0.7–6 cm). The duration of follow-up was averagely (22.1±8.6) months (7–40 months) with a median duration of 18 months. All the patients had been pathologically diagnosed definitely. The general condition of patients was shown in the Table 1. The study was approved by the ethic committee of the Affiliated Hospital of Nantong University (No. 2013-001).
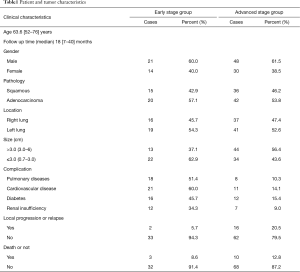
Full table
Therapeutic method
Instrumentation
Siemens Sensation 16-row spiral CT machine (Germany) was used for guidance in this study. The MWA therapeutic device was used for treatment. There are two kinds of he microwave electrodes with different specifications, one was with 1.1 cm anterior pole and 15 cm needle, the other was with 1.1 cm anterior pole and 10 cm needle. The product registration number was State Food and Drug Administration (Approval) No. 3250282, 2011. The transmitting frequency of microwave was set to be 2,450 MHz, and the maximum output power was set to be 100 W. The length of microwave antenna ranged from 100 to 180 mm. The outer diameter of microwave antenna ranged from 14 to 20 G. Its tip was shaped in long conicalness. A water circulation cooling system was used to reduce the surface temperature of antenna.
Preoperative preparation
The patient underwent a preoperative examination of blood coagulation and heart and lung function. The patients with a bleeding tendency accepted preventive homeostasis through the vitamin K and reptilase before and after the operation. CT examination was conducted simultaneously to understand the detailed situation of lesion. A fasting was initiated 12 hours before the operation. A muscular injection of diazepam for 10 mg and morphine for 10 mg was administered 0.5 hour before the operation, and an intravenous injection of flurbiprofen axetil for 50 mg was administered for a preemptive analgesia 15 minutes before the operation. Intravenous injection of flurbiprofen axetil for 50 mg was administered 8 hours after the operation.
Course of operation
According to the preoperative CT image of patient, the focal position, size, shape and its relationship with adjacent organs had been determined to choose the optimal puncture point, puncture path and proper microwave antenna and to set the power and time of MWA. Then under the guidance of CT, the tip of microwave antenna was sent into the focus by puncture for MWA therapy. The ablation power was set as 60–70 W, and the ablation time was determined according to the tumor size, usually set as 4–10 minutes. After the ablation was finished, a needle passage ablation was conducted, and then the power source was shut down. Finally, the MWA antenna was withdrawn, and a local disinfection and bind-up was conducted. A CT scan was conducted immediately after ablation to observe if there is bleeding and other conditions. If the patient had a normal blood pressure, heart rate and blood oxygen saturation and experienced no hemoptysis, chest distress, dyspnea and other symptoms, then he would be allowed to return to the ward.
Evaluation of curative effect and complication
CT scan was conducted to all the patients who received MWA therapy within 48 hours after operation in order to observe the focal condition and find out if there is a presence of complication. An improved Response Evaluation Criteria in Solid Tumors (RECIST) was used to evaluate the curative effect of patients in this group (17,18). RECIST consisted of four levels, complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Local progression or relapse indicates that a tumor is enlarged or a new tumor is present. Through a regular CT observation, this study evaluated the local tumorous curative effect. Meanwhile, the complication of ablation therapy was evaluated according to the criteria established by the international ablation working group in 2009, which consisted of three levels: primary complication, mild complication and side reaction (19).
Statistical analysis
SPSS 17.0 software was used to statistically analyze the data. The χ2 test was used for inter-group qualitative data comparison, and the difference is believed to be statistically significant when P<0.05. Kaplan-Meier method was used to compute the estimated survival time and survival curve, and Wilcoxon test with P<0.05 was believed to be statistically significant.
Results
MWA was used to treat the tumor lesion of 113 patients with lung cancer who were in our hospital. The median diameter of tumors was 3.1 cm (0.7–6 cm). Follow-up were paid to all the patients who received MWA therapy. The duration of follow-up was 7–40 months, and the median follow-up duration was 18 months. All patients with lung cancer experienced MWA therapy, and their tumors were found to have vacuolization, lower density and much smaller distinct shrinkage of tumor size with varying degrees. Generally speaking, MWA is an effective, safe and minimally invasive treatment for the patients with lung.
Curative effects
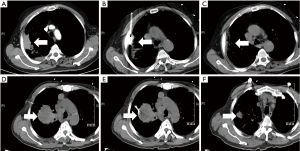
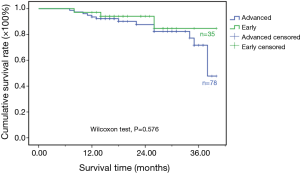
All of the patients with lung cancer underwent MWA therapy were found to have vacuolization, lower density and smaller tumor size with varying degree (see Figure 1 for the typical case). Within the 18-month of median follow-up, the local progression rate or relapse rate of the whole group was 18/113 (15.9%). The counterpart of patients in the early-stage group was 2/35 (5.7%), and the diameter of tumors in the two patients experienced local progression or relapse was more than 3 cm. The local progression rate or relapse rate of patients in advanced-stage group was 16/78 (20.5%), wherein, 13 cases (81.3%, 13/16) of local progression or relapse occurred to the patients with a tumorous diameter of more than 3 cm (Table 2). This result indicated that the patients in advanced-stage group were vulnerable to local progression or relapse, and the tumorous with greater diameter had higher incidence of local relapse. No mortality occurred among the patients in whole group within 30 days after MWA therapy. The survival rate of patients in early-stage group at first, second, third year was respectively 97.1%, 94.1% and 84.7%. The counterpart of advanced-stage group was respectively 93.6%, 87.7% and 71.7%. The difference in survival rate between both groups was not statistically significant (P=0.576) (Figure 2).

Full table
Complication and side effect
Of the 113 patients in this group, 31 patients (27.4%) showed a sign of post-ablation syndrome within the first three days after receiving the ablation therapy, namely, fever, feebleness, nausea, emesis and other symptoms. Non-steroidal antipyretic, analgesics and anti-inflammatory drugs were administered immediately for symptomatic treatment. Twelve patients experienced pneumothorax (10.6%), and were cured by intrathoracic cannula through closed drainage. Nine (8.0%) patients developed pleural effusion. Eleven (9.7%) patients developed hemoptysis, and were stopped effectively by an administration of conventional hemostatic drug. Eight (7.1%) patients experienced pneumonia, and were treated effectively by antibiotic therapy. Twenty-two (19.5%) patients in this study developed a moderate or severe pain during the operation. Six (5.3%) of them experienced severe pain after operation, and they were alleviated by administration of analgesics for symptomatic treatment. No intraoperative and perioperative death occurred among the 113 patients in this study.
Discussion
MWA is an emerging technology in the field of tumor thermal therapy, and it is applicable for the thermal ablation therapy for different kinds of tumors. Its principle is to put the microwave antenna into the tumorous focus by puncture through the skin under the guidance of image technology, and then the polar molecules and charged particles in the tumorous focus would develop a violent rotation due to the influence of electromagnetic radiation field of microwave. As a result, an effect of generation of heat by friction would be produced, which can increase the temperature of tumorous tissues up to 70–160 °C within a short time, and it is enough to result in a degeneration of protein in the tumor, making the tumor cells produce a coagulative necrosis, thus achieving the goal of treating tumor (20,21).
Compared with the previous RFA technology, MWA has more advantages in terms of treatment on lung tumor (22-24). MWA uses a higher frequency of electromagnetic radiation, and it is usually from 915 MHz to 2.45 GHz. MWA can maintain higher temperature in the tumor, which can ablate the large-sized tumor with a short duration of ablation, and multiple MWA antennas can also be used simultaneously. Thus, MWA has already become one of the effective means to treat the lung cancer in a non-surgical manner (25). All the patients in this study underwent an ablation therapy with a microwave frequency of 2,450 MHz, and obtained good curative effects. A proper extension of time during the microwave therapy has become a method widely used clinically to ablate the tumor with a larger scope. Within a certain range of time, the longer the ablation, the greater the diameter of ablation would be. When a relatively large tumor is to be ablated clinically, the microwave power can be properly elevated on the basis of extension of time, so as to achieve better ablation effect (26,27). The ablation power used in this study was 60–70 W, and the ablation duration was dependent on the tumor size, usually set as 4–10 minutes. A good effect has been achieved, and all the tumors of patients in this group were found to have vacuolization, lowered density and distinct shrinkage of tumor size and even disappearance after ablation therapy.
The follow-up duration of the patients in this study was averagely (22.1±8.6) months, and median follow-up duration was 18 months. The results showed that MWA had a good local control rate in treating lung cancer, and its local progression or relapse rate was only 15.9%. Cheng found that MWA therapy can reduce the local relapse rate of lung cancer down to 20% (8). The local progression or relapse rate of patients in early-stage group was 2/35 (5.7%), and the diameter of tumors in the two patients experienced local progression or relapse was more than 3 cm. The local progression rate or relapse rate of patients in advanced-stage group was 20.5%, wherein, 81.3% of local progression or relapse occurred to the patients with a tumorous diameter of more than 3 cm. The study of Sun et al. showed that the local relapse rate of IIIB-IV lung cancer treated by MWA therapy was 26.8% (28). These results indicated that the patients in advanced-stage group were vulnerable to local progression or relapse, and the greater the tumorous diameter, the more possible local progression or relapse would happen. So we can know that MWA has a better local control rate over the early-stage tumor with a small diameter, while with regard to the tumor with a great diameter, it might have a poor local control rate.
There are many reasons for this phenomenon, for instance, oversize of tumor and irregular shape would lead to the failure of ablation antenna to kill all the tumor cells and a residual of tumor cell. Furthermore, there are more and bigger vessels in the greater tumors and the vessel itself had an effect of thermal deposition. Moreover, the failure to thoroughly kill the tumor cells might be due to a metastasis with a varying degree, which resulted in a residual of tumor cell (29). Consequently, as for the tumor with a greater size, a combination with other therapies should be recommended, such as a combination of conformal radiotherapy and chemotherapy and other therapeutic measures after the application of MWA (30,31).
No death occurred within 30 days after MWA therapy was administered to all the patients in the group. The survival rate of the patients in early-stage group at first, second, third year was respectively 97.1%, 94.1% and 84.7%. The counterpart of advanced-stage group was respectively 93.6%, 87.7% and 71.7%, and the difference in survival rate between both groups was not statistically significant (P=0.576), which might be too short for us to follow up. Pneumothorax was the most common complication after MWA therapy. Although the pneumothorax had a high incidence, its symptom was usually mild. So the clinical treatment was unnecessary. Other common complications included hemoptysis, pleural effusion, pneumonia and so on, all of which could be controlled by symptomatic therapy and effective antibiotic therapy. About twenty percent of patients developed an intraoperative pain of a moderate or higher degree, which can be alleviated by administration of analgesics for symptomatic treatment. No intraoperative and perioperative death occurred in this group, which indicated that MWA not only had a low incidence of complication, but also could be effectively controlled. Therefore, MWA had a higher safety for therapy for lung cancer.
In conclusion, the curative effect of CT-guided MWA technology was ensured, and it could effectively control the tumor progression with less adverse reactions and higher safety. So it is promising to be one of the preferred therapeutic methods for patients with lung cancer who are intolerant to surgical treatment.
Acknowledgements
Funding: This study was funded by national science and technology support program (2012BAI15B08).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethic committee of the Affiliated Hospital of Nantong University (No. 2013-001).
References
- Salgia R, Hensing T, Campbell N, et al. Personalized treatment of lung cancer. Semin Oncol 2011;38:274-83. [Crossref] [PubMed]
- Amann A, Corradi M, Mazzone P, et al. Lung cancer biomarkers in exhaled breath. Expert Rev Mol Diagn 2011;11:207-17. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Ding N, Mao Y. Advances in Lymph Node Metastasis and the Modes of Lymph Node Dissection in Early Stage Non-small Cell Lung Cancer. Zhongguo Fei Ai Za Zhi 2016;19:359-63. [PubMed]
- Bonichon F, Godbert Y, Gangi A, et al. PET/Computed Tomography and Thermoablation (Radiofrequency, Microwave, Cryotherapy, Laser Interstitial Thermal Therapy). PET Clin 2015;10:519-40. [Crossref] [PubMed]
- Shahzad Y, Louw R, Gerber M, et al. Breaching the skin barrier through temperature modulations. J Control Release 2015;202:1-13. [Crossref] [PubMed]
- Ierardi AM, Lucchina N, Petrillo M, et al. Systematic review of minimally invasive ablation treatment for locally advanced pancreatic cancer. Radiol Med 2014;119:483-98. [Crossref] [PubMed]
- Cheng M, Fay M, Steinke K. Percutaneous CT-guided thermal ablation as salvage therapy for recurrent non-small cell lung cancer after external beam radiotherapy: A retrospective study. Int J Hyperthermia 2016;32:316-23. [Crossref] [PubMed]
- Pacella CM, Papini E. Image-guided percutaneous ablation therapies for local recurrences of thyroid tumors. J Endocrinol Invest 2013;36:61-70. [Crossref] [PubMed]
- Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg 2011;98:1210-24. [Crossref] [PubMed]
- Ikemoto T, Shimada M, Yamada S. Pathophysiology of recurrent hepatocellular carcinoma after radiofrequency ablation. Hepatol Res 2017;47:23-30. [Crossref] [PubMed]
- Ierardi AM, Floridi C, Fontana F, et al. Microwave ablation of liver metastases to overcome the limitations of radiofrequency ablation. Radiol Med 2013;118:949-61. [Crossref] [PubMed]
- Liu Y, Li S, Wan X, et al. Efficacy and safety of thermal ablation in patients with liver metastases. Eur J Gastroenterol Hepatol 2013;25:442-6. [Crossref] [PubMed]
- Baisi A, De Simone M, Raveglia F, et al. Thermal ablation in the treatment of lung cancer: present and future. Eur J Cardiothorac Surg 2013;43:683-6. [Crossref] [PubMed]
- Fan W, Li X, Zhang L, et al. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. AJR Am J Roentgenol 2012;198:W46-50. [Crossref] [PubMed]
- Poulou LS, Botsa E, Thanou I, et al. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol 2015;7:1054-63. [Crossref] [PubMed]
- Nishino M, Jackman DM, Hatabu H, et al. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol 2010;195:W221-8. [Crossref] [PubMed]
- Watanabe H, Kunitoh H, Yamamoto S, et al. Effect of the introduction of minimum lesion size on interobserver reproducibility using RECIST guidelines in non-small cell lung cancer patients. Cancer Sci 2006;97:214-8. [Crossref] [PubMed]
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009;20:S377-90. [Crossref] [PubMed]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25 Suppl 1:S69-83. [Crossref] [PubMed]
- Smith SL, Jennings PE. Lung radiofrequency and microwave ablation: a review of indications, techniques and post-procedural imaging appearances. Br J Radiol 2015;88:20140598. [Crossref] [PubMed]
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol 2009;38:135-43. [Crossref] [PubMed]
- Wolf FJ, Aswad B, Ng T, et al. Intraoperative microwave ablation of pulmonary malignancies with tumor permittivity feedback control: ablation and resection study in 10 consecutive patients. Radiology 2012;262:353-60. [Crossref] [PubMed]
- Schneider T, Heussel CP, Herth FJ, et al. Thermal ablation of malignant lung tumors. Dtsch Arztebl Int 2013;110:394-400. [PubMed]
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol 2011;79:124-30. [Crossref] [PubMed]
- Hernández JI, Cepeda MF, Valdés F, et al. Microwave ablation: state-of-the-art review. Onco Targets Ther 2015;8:1627-32. [PubMed]
- Lubner MG, Brace CL, Ziemlewicz TJ, et al. Microwave ablation of hepatic malignancy. Semin Intervent Radiol 2013;30:56-66. [Crossref] [PubMed]
- Sun YH, Song PY, Guo Y, et al. Effects of microwave ablation or its combination with whole-body chemotherapy on serum vascular endothelial growth factor levels in patients with stage IIIB/IV NSCLC. Genet Mol Res 2015;14:10015-25. [Crossref] [PubMed]
- Zhang NN, Lu W, Cheng XJ, et al. High-powered microwave ablation of larger hepatocellular carcinoma: evaluation of recurrence rate and factors related to recurrence. Clin Radiol 2015;70:1237-43. [Crossref] [PubMed]
- Das M, Abdelmaksoud MH, Loo BW Jr, et al. Alternatives to surgery for early stage non-small cell lung cancer-ready for prime time? Curr Treat Options Oncol 2010;11:24-35. [Crossref] [PubMed]
- Jahangeer S, Forde P, Soden D, et al. Review of current thermal ablation treatment for lung cancer and the potential of electrochemotherapy as a means for treatment of lung tumours. Cancer Treat Rev 2013;39:862-71. [Crossref] [PubMed]


