Ablation of atrial fibrillation: single-shot techniques poised to dominate rhythm control strategies/the future is here
Introduction
Atrial fibrillation (AF) is the commonest cardiac arrhythmia associated with higher mortality and morbidity compared to those without AF, accounting for 20–30% of all strokes, impairing quality of life, leading to left ventricular dysfunction in 20–30% of all AF patients and implicated in cognitive decline even in anticoagulated patients (1). Due to disappointing results of drug therapies to restore and maintain sinus rhythm, clinicians are slowly but steadily resorting to catheter ablation techniques over the recent years, as ablation technology has emerged as a more effective and practical approach to AF management, albeit at an increased procedural risk (2), in an attempt to decrease the AF disease burden and its attendant exuberant health expenditure.
Pulmonary vein (PV) isolation (PVI) is the cornerstone of all AF ablation techniques (1,3). This is currently effected via application of radiofrequency (RF) lesions in a circular pattern around the antrum of the four PVs, while avoiding lesions inside the veins to prevent development of PV stenosis. Other additional methods, that have been mostly applied to patients with persistent AF, comprise linear lesions in the left atrium, ablation of areas with fragmented local electrograms and ablation of ganglionated plexi, which however have not practically enhanced the ablation success achieved by PVI (4).
Conventional approaches to AF ablation employing point-by-point RF ablation with use of irrigated catheters are complex, lengthy and risky procedures which require double transseptal puncture or single-puncture double transseptal catheterization techniques with a consequent increase in risk and duration of the procedure (3). Furthermore, extensive left atrial mapping with the use of multiple catheters is required. During point-by-point ablation, gaps in the ablation lines are inevitable. The potential complications of damage to the adjacent esophagus and PV ostial stenosis are additional risks. All these drawbacks result in a slow learning curve, large dependence of procedural outcomes on operator dexterity and experience, patient inconvenience and greater resource utilization. Thus, more simplified, swift and less expensive techniques for AF ablation have been sought.
Over the recent years, the advent of single-shot techniques has ushered in a new era in the AF ablation approach and rhythm control strategies (5,6). These techniques make it easier to navigate the heart and have the potential to decrease the threshold for and expand the access to rhythm-control therapies, that may furnish further reduction of the AF disease burden.
Single-shot techniques
Two recently developed technologies provide the single-shot approach to PVI, the multi-electrode circular ablation catheter and the cryoballoon catheter (5-9). Both these methods have emerged as valid alternatives to point-by-point RF ablation with the added advantage of combining mapping and PVI in a single-shot and shorter procedure.
Circular multi-electrode catheter technique
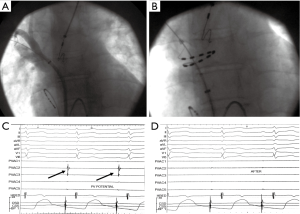
The Pulmonary Vein Catheter (PVAC) (Medtronic Inc, Minneapolis, MN, USA) is a 9 Fr, over-the-wire, circular-shaped, decapolar pacing, mapping and ablation catheter with an adjustable 25 mm diameter array at the distal portion (5,10). The platinum electrodes are 3-mm long with 3-mm spacing. The catheter is steerable and deflectable bidirectionally, facilitating correct positioning at the PV antrum. In addition, with use of the guidewire, the electrode array can be extended to assume a spiral configuration that enables mapping inside the PV. This technology uses both a new catheter design and a specific mode of RF energy (duty-cycled phased RF energy). Each electrode has a thermocouple giving temperature feedback to an individual subunit of the RF generator that continuously regulates and adapts the power output to the electrode to maintain the target temperature (~60 °C), avoiding its overshoot. The generator uses RF energy in a special way, by providing duty-cycled alternating unipolar and bipolar current. The unipolar current flows between the electrodes and the indifferent electrode (backplate) to create deeper lesions. The bipolar current flows between adjacent electrodes to create lesion continuity between the electrodes, thus filling the gaps and resulting in a circular lesion. In addition, the generator can mix the phase differences of the duty-cycled energy on individual electrodes in specific ratios of bipolar to unipolar energy. At a 4:1 ratio (80% bipolar/20% unipolar), the lesion depth is smaller (~3 mm) and preferred for PVI to avoid perforation of the thin PV antrum and/or heating of the tissue of the adjacent structures. Use of the PVAC requires a single transseptal puncture and one transseptal sheath to guide the ablation catheter. During RF delivery, real-time electrogram signal monitoring is not feasible. Signal assessment is made between applications.
Both the acute and long-term results with use of the PVAC appear favorable. In 98 patients (aged 59±9 years), PVAC ablation was successful in isolating all (100%) 369 PVs with a mean of 27±7 RF applications, total procedural time 84±29 min, and fluoroscopy time 18±8 min, without procedure-related complications (11). Follow-up after 6 months without antiarrhythmic drugs showed freedom from AF in 83% of patients. Similarly, among 88 patients (mean age 58±11 years) with symptomatic paroxysmal AF, 338 of 339 targeted PVs (99%) were isolated with the PVAC with a mean of 24±9 RF applications per patient, procedure time of 125±28 minutes, and fluoroscopy time of 21±13 minutes, with no procedure-related complications (12). Freedom from AF off antiarrhythmic drugs was reported in 80% of patients at 1 year.
AF ablation with the PVAC catheter also shows long-term safety and effectiveness with relatively short procedure times. Among 429 patients (mean age 60±12 years, 58% men, 68% PAF, 32% persistent AF; 75 patients having two procedures; mean procedure time: 62±15 min; 2.1% complication rate), over 22±5 months, freedom from AF recurrence was 68.5% (13). In a smaller study, among 77 with paroxysmal AF submitted to PVAC ablation, after a single procedure at a mean follow-up of 55±11 months, 54/77 (70.1%) patients were free of symptomatic AF (14).
Comparative studies between the circular catheter technique and the conventional irrigated catheter technique have yielded comparable results. Among 161 consecutive patients with symptomatic paroxysmal or persistent AF undergoing PVI via PVAC (n=79) or conventional ablation (n=82), at 3 years of follow-up, single-procedure success without antiarrhythmic drugs was comparable between the two groups (65% vs. 55%, P=NS). The majority of recurrences occurred during the first year (79% vs. 70%, P=NS) (15). The annual rate of very late recurrence (>1 year) was also similar in both groups (10.5% vs. 15%, P=NS).
Comparative studies between the PVAC and the cryoballoon catheter have also yielded similar outcomes. Among 110 AF patients, randomized to either the cryoballoon or the PVAC catheter, complete PVI was achieved in 98% vs. 93% patients in the cryoballoon and PVAC group, respectively, with complication rates of 8% vs. 2% (P=0.2) (16). Freedom from AF, without antiarrhythmic drugs, after a single ablation procedure was seen in 46% in the cryoballoon vs. 34% after 12 months (P=0.2). Procedure times were comparable, but fluoroscopy time was shorter for the cryoballoon (32±16 min) than for the PVAC procedures (47±17 min) (P<0.001). It should be noted that these data come from use of the first-generation technologies.
An observed higher rate of silent cerebral infarcts on magnetic resonance imaging with this technology (17) led to initial withdrawal of the PVAC catheter and after technological improvements it has been re-introduced into the clinical arena (18). However, the newer data with the second-generation catheter (containing 9 gold electrodes) are not adequate as yet to definitely document its comparative efficacy and safety with the other technologies (18,19). There ensued the development of another type of irrigated circular ablation catheter (nMARQ catheter, Biosense Webster Inc, Diamond Bar, CA, USA), with good initial results (20,21), however, due to observed fatal complications from atrio-esophageal fistula, this catheter was recalled from the market in 2015 (22). Attention has been consequently shifted to the cryoballoon catheter technology.
Cryoballoon catheter technique
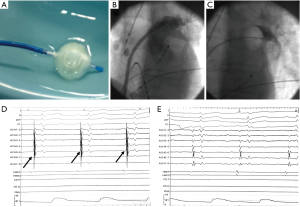
Cryoenergy applied via a balloon catheter introduced into the left atrium via a single transseptal puncture has emerged as a more practical, versatile and effective technique over the recent years, aspiring to become the dominant ablation method promising to facilitate PVI, decrease the complication rate, shorten the duration of the procedure and probably enhance the success rate of ablation (6). It is a single-shot technique as the balloon catheter also accommodates a small circular mapping catheter introduced via the balloon catheter that can map the PVs and also confirm or even provide real-time monitoring of successful ablation with its newer generation versions. In its newer version (second generation), the balloon catheter appears to be able to produce more effective and durable transmural lesions around the PV antrum with shorter, 3- vs. 4-minute freezing, without the need for a bonus application which in the beginning was routine practice. Newer data provide guidance to a more practical and effective strategy while applying this freezing technique (23).
Need for bonus cryoenergy applications and predictors of successful PVI and favorable clinical outcome
With regard to the need for bonus applications after achieving PVI with cryoablation, a recent study suggested that a short time (<43 sec) required to achieve PVI may obviate the need for bonus freezing (23). According to this study (ICE-T trial), which randomized AF patients to empiric bonus PV freezing (n=50; control group) or an individualized freezing approach based on time-to-isolation (n=50), monitoring time-to-isolation was used as a guide to titrate ablation energy, expedite the procedure, and obtain a high (88%) success rate and clinical outcome of single- shot PVI. The authors of this study suggest that when time to PVI exceeds 43 sec, this denotes an insufficient freeze, which should be terminated early and a new balloon position should be sought for a more effective application.
Time to PVI has been found to be an important predictor of successful and durable PVI in other studies, as well (24). Hence, the need to have improved technology to be able to continuously monitor real-time PV potential recordings during cryothermic energy application is crucial. With current technology, this may not be feasible at all times, as the recording circular catheter cannot always be kept during freezing in proximal positions required for this kind of monitoring. Newer technology with shorter tipped (third generation) balloons may indeed render this possible (25); however, further technical details for more versatile and effective balloon catheters may have to be worked out before these balloons become more widely available.
Other predictors of successful and durable PVI include a minimal temperature of <–51 °C and balloon warming time or interval thaw time at 0 °C >10 sec, which appear to reduce the possibility of PV reconnection (26,27). Different sites of transseptal puncture have no influence in grades of PV occlusion, rates of PVI, mid-term outcome and rates of complications during cryoablation (6). Imaging techniques, such as intracardiac echocardiography, guiding exact balloon placement may predict acute ablation success and prevent acute narrowing of PV ostia. Total duration of cryoenergy application has not been shown to affect procedural success, with similar success rates obtained with 3- and 4-minute applications (28). Importantly, a single 3-minute freezing application with no need for a “bonus” strategy appears to suffice provided that very low nadir temperatures and/or short times to PVI have been obtained during the cryoenergy application. Thus, shorter time of freezing application can expedite the procedure and lead to lower radiation exposure without compromising procedure efficacy. On the other hand, longer observational time and adenosine challenge at the end of the procedure have been recommended to detect PV reconnections; however, the incidence of such spontaneous and adenosine-induced PV reconnection following ablation with the second generation cryoballoon is very low (~4%) and thus not worth to prolong the procedure for this reason (6).
In summary, obtaining good PV occlusion with the balloon that will achieve nadir temperature <−51 °C and rewarming time >10–28 sec, can prevent acute reconnections, thus obviating the need for extra waiting time and adenosine challenge that will prolong the procedure. Moreover, monitoring real-time PV potential recordings during application of cryoenergy and observing their disappearance within ~40–70 sec seems to ensure successful and durable PVI, also obviating the need for bonus applications with their attendant procedural delay and added risk. All these parameters have also been shown to predict favorable clinical outcomes.
Complications
Although initial reports of complications at the worrisome percentages of 4–6% made clinicians hesitant in referring patients to the conventional point-by-point RF ablation technique, technical developments over the years and the increased operator experience have provided much improved results with lessened complication rates (6,29,30). The advent of cryoballoon ablation seems to have contributed to further drastically curtail these rates, with complications currently reported at rates around 2% according to more recent reports (31). The most feared complication relates to cardiac tamponade which can necessitate surgical intervention and can rarely lead to fatal outcome. Another dreaded complication, the development of an atrio-esophageal fistula, which is usually fatal, has also been observed more rarely with the cryoablation approach compared to the RF technique (32). The higher rate of phrenic nerve injury with PVI of the right PVs using cryoenergy appears to be manageable with monitoring of the phrenic nerve function by applying phrenic nerve pacing via a catheter positioned at the right superior vena cava. Furthermore, the reversibility of this injury over time in the majority of patients is very encouraging (33).
Further perspectives
An important aspect of AF ablation relates to operator proficiency and dexterity in performing transseptal catheterization safely (34). Then, careful and gentle handling and maneuvering the ablation tools inside the left atrium and the PVs is of utmost importance to avoid further complications.
Single-shot techniques compare well with the conventional point-by-point RF ablation approach, although the latter has recently received a boost with the advent of contact force monitoring during ablation, albeit without managing to surpass cryoablation in terms of procedure duration, which remains shorter with the second generation cryoballoon (35).
Of the two single-shot techniques, cryoballoon ablation has taken a lead mostly due to technical issues that have emerged with the circular catheters, and also the difference in energy source that might be providing an edge over conventional RF energy (16,34,36-39). According to our own experience with these two techniques (Figures 1,2, Table 1), the circular catheter was employed during the early years of its availability and replaced the conventional irrigated catheter in our laboratory, while the cryoballoon was adopted later when the circular catheter was withdrawn due to reports of silent cerebral infarcts attributed to technical issues. With the availability of the second generation cryoballoon catheter and the perceived convenience that it offered and its probable enhanced efficacy, we have since stayed with this technology and are currently performing all AF ablation procedures with the cryotechnique, even for all repeat procedures in patients having recurrences. Clinical success has remained approximately the same (>80%) over a mean follow-up of about 2 years, however, shorter radiation exposure and duration of the procedure are notable (Table 1), although this may relate to a learning curve effect with the single-shot techniques. Importantly, no clinical events of cerebral infarcts, PV stenoses or esophageal lesions have been observed with either technique.
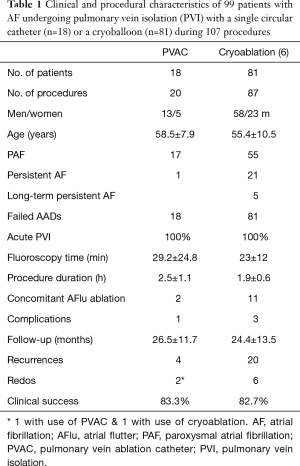
Full table
The ability to monitor real-time and document successful PVI during the freezing process will obviate the need for extra freezing applications, reduce the duration and risk of the procedure and increase the success rate of the single-shot technique. Third generation balloons have been developed with shorter balloon tips to provide such monitoring (40), however, there are still technical problems that have prevented their wider availability.
It is estimated that AF triggers emanate from the PVs in 80% of the cases, while in 20% extrapulmonary sources may be responsible for the arrhythmia (41,42). The ability to also use the cryoballoon for ablating extrapulmonary sources of AF triggers offers some promise that this may turn out to be the dominant rhythm control strategy. Indeed, the balloon was employed to successfully isolate the superior vena cava in one case (43) and in another case a persistent left superior vena cava (44). Furthermore, the left atrial appendage has also been successfully isolated with use of the cryoballoon (45); whether this is going to be an appropriate approach for AF ablation remains to be seen in future studies.
Comparative studies between conventional RF ablation and cryoballoon ablation have indicated comparable results, although they all emphasize the shorter procedure duration incurred with the cryotechnique (46-48). Indeed, a recent meta-analysis of 38 studies, 9 randomized controlled trials (RCTs) and 29 non- RCTs, comprising 15,496 patients, indicated that cryoballoon ablation was more beneficial in terms of procedural time, complications without phrenic nerve injury, and recurrences for paroxysmal AF (48). However, the newer contact-force mode of RF and second generation cryoballoon ablation showed similar clinical benefits. Real-world data also confirm the excellent safety profile and satisfactory acute success rate and short procedural times with cryoballoon ablation in large patient cohorts (49). Furthermore, other important advantages of the cryotechnique relate to the learning curve and method complexity; recent data indicate that the learning curve with cryoballoon ablation is steep, with only 20–30 cases required for an inexperienced operator to acquire proficiency (50), while other studies have indicated that the cryoballoon seems to be less operator-dependent and more reproducible than RF in the setting of paroxysmal AF ablation. Finally, one crucial issue of all ablation techniques relates to recurrences and need for repeat procedures, which culminate into high percentages of 30–50%. The cryotechnique appears promising in lowering these percentages (48,51-53).
A most recent review of ablation efficacy (ESC-EHRA Atrial Fibrillation Ablation Long-Term registry), reporting on procedures from 104 centers in 27 European countries performed during a 3-year period (April 2012-April 2015), presents a guarded and cautious overall view of AF ablation with use of conventional techniques in 77% of cases (54). Among 3,593 patients (median age 59 years) undergoing AF ablation, PVI was achieved in 95–97%, inhospital complications occurred in 7.8%, with cardiac perforation occurring in 1.3%, and one patient died due to an atrio-esophageal fistula. One-year follow-up, performed in 88.6%, showed a success rate with or without antiarrhythmic drugs in 73.6%; a significant portion (46%) was still on drugs. Late complications included 14 additional deaths (4 cardiac, 4 vascular, 6 other causes) and 333 (10.7%) other complications.
The above report curtails one’s enthusiasm that has prevailed over the recent years with the purported advances made in the AF ablation field and produces serious scepticism. However, this suboptimal success rate and considerable complication rate of the conventional approach may hopefully change in the future with the wider application of single-shot techniques, such as the cryoballoon ablation approach, as long as they remain safe and effective. Although several initial studies report very encouraging results, one still needs to await the results and long-term outcome data from larger prospective randomized studies before fully adopting the herein presented optimistic view of this promising technology. Nevertheless, many centers appear to be embracing this newer and simpler rhythm-controlling approach.
Acknowledgements
The author thanks the Staff of the Electrophysiology Laboratories at Evagelismos and Ippokrateio Hospitals for their invaluable assistance with the procedures.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-962. [Crossref] [PubMed]
- Hakalahti A, Biancari F, Nielsen JC, et al. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace 2015;17:370-8. [Crossref] [PubMed]
- Haegeli LM, Calkins H. Catheter ablation of atrial fibrillation: an update. Eur Heart J 2014;35:2454-9. [Crossref] [PubMed]
- Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812-22. [Crossref] [PubMed]
- Nobre Menezes M, Cortez-Dias N, Carpinteiro L, et al. One-Shot Ablation For PV Isolation. J Atr Fibrillation 2014;7:1111. [PubMed]
- Georgiopoulos G, Tsiachris D, Manolis AS. Cryoballoon ablation of atrial fibrillation: a practical and effective approach. Clin Cardiol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Deneke T, Mügge A, Balta O, et al. Treatment of persistent atrial fibrillation using phased radiofrequency ablation technology. Expert Rev Cardiovasc Ther 2011;9:1041-9. [Crossref] [PubMed]
- Mönnig G, Eckardt L. Multielectrode Pulmonary Vein Ablation Catheter (PVAC(®)): current data on results and risks. Herzschrittmacherther Elektrophysiol 2014;25:236-40. [Crossref] [PubMed]
- Aryana Ms Md Fhrs A, Bowers Ms Md MR, O'Neill Md Fhrs AP. Outcomes Of Cryoballoon Ablation Of Atrial Fibrillation: A Comprehensive Review. J Atr Fibrillation 2015;8:1231. [PubMed]
- Kiss Md PhD A, Sándorfi Md G, Nagy-Baló Md PhD E, et al. Phased RF Ablation: Results and Concerns. J Atr Fibrillation 2015;8:1240. [PubMed]
- Boersma LV, Wijffels MC, Oral H, et al. Pulmonary vein isolation by duty-cycled bipolar and unipolar radiofrequency energy with a multielectrode ablation catheter. Heart Rhythm 2008;5:1635-42. [Crossref] [PubMed]
- Wieczorek M, Hoeltgen R, Akin E, et al. Results of short-term and long-term pulmonary vein isolation for paroxysmal atrial fibrillation using duty-cycled bipolar and unipolar radiofrequency energy. J Cardiovasc Electrophysiol 2010;21:399-405. [Crossref] [PubMed]
- Nardi S, Argenziano L, Cappato R, et al. Ablation of paroxysmal and persistent atrial fibrillation with multielectrode phased radiofrequency duty-cycled catheters: long-term results from a large cohort of patients. J Cardiovasc Med (Hagerstown) 2013;14:879-85. [Crossref] [PubMed]
- Lepillier A, Copie X, Lascault G, et al. A 5-year clinical follow-up after duty-cycled phased RF ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2017;48:327-31. [Crossref] [PubMed]
- De Greef Y, Buysschaert I, Schwagten B, et al. Duty-cycled multi-electrode radiofrequency vs. conventional irrigated point-by-point radiofrequency ablation for recurrent atrial fibrillation: comparative 3-year data. Europace 2014;16:820-5. [Crossref] [PubMed]
- Malmborg H, Lönnerholm S, Blomström P, et al. Ablation of atrial fibrillation with cryoballoon or duty-cycled radiofrequency pulmonary vein ablation catheter: a randomized controlled study comparing the clinical outcome and safety; the AF-COR study. Europace 2013;15:1567-73. [Crossref] [PubMed]
- Gaita F, Leclercq JF, Schumacher B, et al. Incidence of silent cerebral thromboembolic lesions after atrial fibrillation ablation may change according to technology used: comparison of irrigated radiofrequency, multipolar nonirrigated catheter and cryoballoon. J Cardiovasc Electrophysiol 2011;22:961-8. [Crossref] [PubMed]
- Gal P, Buist TJ, Smit JJ, et al. Effective contact and outcome after pulmonary vein isolation in novel circular multi-electrode atrial fibrillation ablation. Neth Heart J 2017;25:16-23. [Crossref] [PubMed]
- Weber S, Höher M, Schultes D. First results and follow-up of a second-generation circular mapping and ablation catheter. J Interv Card Electrophysiol 2016;47:213-9. [Crossref] [PubMed]
- Shin DI, Kirmanoglou K, Eickholt C, et al. Initial results of using a novel irrigated multielectrode mapping and ablation catheter for pulmonary vein isolation. Heart Rhythm 2014;11:375-83. [Crossref] [PubMed]
- Mahida S, Hooks DA, Nentwich K, et al. nMARQ Ablation for Atrial Fibrillation: Results from a Multicenter Study. J Cardiovasc Electrophysiol 2015;26:724-9. [Crossref] [PubMed]
- Vurma M, Dang L, Brunner-La Rocca HP, et al. Safety and efficacy of the nMARQ catheter for paroxysmal and persistent atrial fibrillation. Europace 2016;18:1164-9. [Crossref] [PubMed]
- Chun KR, Stich M, Fürnkranz A, et al. Individualized cryoballoon energy pulmonary vein isolation guided by real-time pulmonary vein recordings, the randomized ICE-T trial. Heart Rhythm 2017;14:495-500. [Crossref] [PubMed]
- Aryana A, Mugnai G, Singh SM, et al. Procedural and biophysical indicators of durable pulmonary vein isolation during cryoballoon ablation of atrial fibrillation. Heart Rhythm 2016;13:424-32. [Crossref] [PubMed]
- Aryana A, Kowalski M, O'Neill PG, et al. Catheter ablation using the third-generation cryoballoon provides an enhanced ability to assess time to pulmonary vein isolation facilitating the ablation strategy: Short- and long-term results of a multicenter study. Heart Rhythm 2016;13:2306-2313. [Crossref] [PubMed]
- Ghosh J, Martin A, Keech AC, et al. Balloon warming time is the strongest predictor of late pulmonary vein electrical reconnection following cryoballoon ablation for atrial fibrillation. Heart Rhythm 2013;10:1311-7. [Crossref] [PubMed]
- Fürnkranz A, Köster I, Chun KR, et al. Cryoballoon temperature predicts acute pulmonary vein isolation. Heart Rhythm 2011;8:821-5. [Crossref] [PubMed]
- Ciconte G, Sieira-Moret J, Hacioglu E, et al. Single 3-Minute versus Double 4-Minute Freeze Strategy for Second-Generation Cryoballoon Ablation: A Single-Center Experience. J Cardiovasc Electrophysiol 2016;27:796-803. [Crossref] [PubMed]
- Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005;111:1100-5. [Crossref] [PubMed]
- Dagres N, Hindricks G, Kottkamp H, et al. Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern? J Cardiovasc Electrophysiol 2009;20:1014-9. [Crossref] [PubMed]
- Mugnai G, de Asmundis C, Ciconte G, et al. Incidence and characteristics of complications in the setting of second-generation cryoballoon ablation: A large single-center study of 500 consecutive patients. Heart Rhythm 2015;12:1476-82. [Crossref] [PubMed]
- John RM, Kapur S, Ellenbogen KA, et al. Atrioesophageal fistula formation with cryoballoon ablation is most commonly related to the left inferior pulmonary vein. Heart Rhythm 2017;14:184-9. [Crossref] [PubMed]
- Guhl EN, Siddoway D, Adelstein E, et al. Incidence and Predictors of Complications During Cryoballoon Pulmonary Vein Isolation for Atrial Fibrillation. J Am Heart Assoc 2016;5:e003724. [Crossref] [PubMed]
- Su W, Kowal R, Kowalski M, et al. Best practice guide for cryoballoon ablation in atrial fibrillation: The compilation experience of more than 3000 procedures. Heart Rhythm 2015;12:1658-66. [Crossref] [PubMed]
- Squara F, Zhao A, Marijon E, et al. Comparison between radiofrequency with contact force-sensing and second-generation cryoballoon for paroxysmal atrial fibrillation catheter ablation: a multicentre European evaluation. Europace 2015;17:718-24. [Crossref] [PubMed]
- Aryana A, Singh SM, Kowalski M, et al. Acute and Long-Term Outcomes of Catheter Ablation of Atrial Fibrillation Using the Second-Generation Cryoballoon versus Open-Irrigated Radiofrequency: A Multicenter Experience. J Cardiovasc Electrophysiol 2015;26:832-9. [Crossref] [PubMed]
- Andrade JG, Khairy P, Dubuc M. Catheter cryoablation: biology and clinical uses. Circ Arrhythm Electrophysiol 2013;6:218-27. [Crossref] [PubMed]
- Andrade JG, Dubuc M, Guerra PG, et al. The biophysics and biomechanics of cryoballoon ablation. Pacing Clin Electrophysiol 2012;35:1162-8. [Crossref] [PubMed]
- Herrera Siklódy C, Deneke T, Hocini M, et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation: comparison of different atrial fibrillation ablation technologies in a multicenter study. J Am Coll Cardiol 2011;58:681-8. [Crossref] [PubMed]
- Pott A, Petscher K, Messemer M, et al. Increased rate of observed real-time pulmonary vein isolation with third-generation short-tip cryoballoon. J Interv Card Electrophysiol 2016;47:333-9. [Crossref] [PubMed]
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [Crossref] [PubMed]
- Ouyang F, Antz M, Ernst S, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation 2005;111:127-35. [Crossref] [PubMed]
- Gonna H, Domenichini G, Conti S, et al. Cryoballoon isolation of the superior vena cava. JACC Clin EP 2016;2:529-31.
- Schneider MA, Schade A, Koller ML, et al. Cryoballoon ablation of paroxysmal atrial fibrillation within the dilated coronary sinus in a case of persistent left superior vena cava. Europace 2009;11:1387-9. [Crossref] [PubMed]
- Bordignon S, Perrotta L, Fürnkranz A, et al. Durable single shot cryoballoon isolation of the left atrial appendage followed by percutaneous left atrial appendage closure. Circ Arrhythm Electrophysiol 2015;8:751-2. [Crossref] [PubMed]
- Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med 2016;374:2235-45. [Crossref] [PubMed]
- Chen YH, Lu ZY, Xiang Y, et al. Cryoablation vs. radiofrequency ablation for treatment of paroxysmal atrial fibrillation: a systematic review and meta-analysis. Europace 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Chen CF, Gao XF, Duan X, et al. Comparison of catheter ablation for paroxysmal atrial fibrillation between cryoballoon and radiofrequency: a meta-analysis. J Interv Card Electrophysiol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Padeletti L, Curnis A, Tondo C, et al. Pulmonary Vein Isolation with the Cryoballoon Technique: Feasibility, Procedural Outcomes, and Adoption in the Real World: Data from One Shot Technologies TO Pulmonary Vein Isolation (1STOP) Project. Pacing Clin Electrophysiol 2017;40:46-56. [Crossref] [PubMed]
- Velagić V, de Asmundis C, Mugnai G, et al. Learning curve using the second-generation cryoballoon ablation. J Cardiovasc Med (Hagerstown) 2016. [Epub ahead of print]. [PubMed]
- Liu XH, Chen CF, Gao XF, et al. Safety and Efficacy of Different Catheter Ablations for Atrial Fibrillation: A Systematic Review and Meta-Analysis. Pacing Clin Electrophysiol 2016;39:883-99. [Crossref] [PubMed]
- Hunter RJ, Baker V, Finlay MC, et al. Point-by-Point Radiofrequency Ablation Versus the Cryoballoon or a Novel Combined Approach: A Randomized Trial Comparing 3 Methods of Pulmonary Vein Isolation for Paroxysmal Atrial Fibrillation (The Cryo Versus RF Trial). J Cardiovasc Electrophysiol 2015;26:1307-14. [Crossref] [PubMed]
- Kuck KH, Fürnkranz A, Chun KR, et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J 2016;37:2858-65. [Crossref] [PubMed]
- Arbelo E, Brugada J, Lundqvist CB, et al. Contemporary management of patients undergoing atrial fibrillation ablation: in-hospital and 1-year follow-up findings from the ESC-EHRA atrial fibrillation ablation long-term registry. Eur Heart J 2017. [Epub ahead of print]. [Crossref] [PubMed]


