Trimodality treatment seems to be the best therapeutic option for malignant mesothelioma patients, achieving an overall survival longer than 20 months in some series (
3-6). The main effect of chemotherapy in this setting seemed to be disease stabilization (40-70%), whereas response rate was about 30%.
In the multimodality approach, the goal of surgery is to provide a macroscopic complete resection defined as the removal of all tumoral lesions without macroscopic residues.
Preoperative chemotherapy based on the association of cisplatin plus pemetrexed achieved 1.3% of radiological complete responses and about 5% of pathological complete responses among patients who underwent extrapleural pneumonectomy (
5,
6). These data underline that induction chemotherapy in mesothelioma patients achieves a lower rate of pathologic complete response compared to other tumor types (e.g. non-small cell lung cancer). Further efforts are needed to improve cytotoxicity of currently used regimens in order to improve the outcome of the affected patients and to get the pathological diagnosis and staging of the disease.
Patients not eligible for radical surgery seem to benefit from carboplatin plus pemetrexed. A phase II trial showed a response rate about 20% (2% complete response, 18% partial response) and a disease stabilization of 47%. Median time to progression was 6.5 months and median overall survival time was 12.7 months (
2), quite similar to the results achieved with the standard regimen of pemetrexed and cisplatin (
1). The regimen was well-tolerated and patients with disease control showed a stabilization or improvement of quality of life.
These evidences underline that carboplatin plus pemetrexed could be a valid option as preoperative chemotherapeutic regimen in patients eligible for a multimodality approach.
So far, no prospective trial with carboplatin plus pemetrexed as induction chemotherapy in the context of a multimodality treatment was performed. We have recently analyzed activity and tolerability of pemetrexed plus carboplatin or cisplatin in the first-line treatment of 54 operable patients with resectable MPM. We showed a response rate of 33% (complete response: 3%, partial response: 30%) in patients treated with pemetrexed/carboplatin (AC) vs 17% (no complete response, 17% of partial response) in patients treated with pemetrexed/cisplatin (AP). Moreover, cumulative non-haematological toxicities and PS worsening were commoner in AP-treated patients, and this could impair the clinical conditions of patients undergoing surgery.
Two surgical approaches are possible in operable MPM patients: extrapleural pneumonectomy in case of advanced locally invasive disease with extensive involvement of visceral pleura and fissures, and pleurectomy/decortication in patients with superficial tumors without large involvement of lung and fissures. Moreover, patients with limited disease and a compromised respiratory function test of the tumor-involved lung could benefit from pneumonectomy.
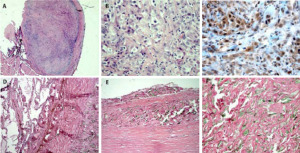
In this particular case, considering the limited stage of disease and the presence of a single nodule-like feature in the interlobar fissure, our patient underwent a parietal pleurectomy with lung decortication.
The surgical specimen of the patient we reported about showed no residual malignancy at the pathological examination, and this seems quite unexpected according to the high chemoresistance of the disease. Favourable factors in this patient were stage II and epithelioid histology. Furthermore, toxicity was moderate and no dose reduction was applied. These factors might have had an effect on improved response to chemotherapy. A good concordance between radiological and pathological response was found: CT-scan showed the persistence of a modest pleural thickening at the rib vertebral angle, but this corresponded to a fibrous-hyaline thickening and focal calcinosis at the pathological examination.
Complete resection allows the pathologist to better describe peculiar biological characteristics of the tumoral sample, in order to select mesothelioma patients with different prognosis and who could benefit from targeted treatments.
The availability of mesothelioma tissue from surgical or bioptical specimens could improve the analysis of anti-apoptotic protein expression, namely Bcl-2 and IAP (inhibitors of apoptosis proteins) family, which seem to be highly expressed in chemo-refractory MPM.
The inhibition of such targets could sensitize mesothelioma cells to apoptosis, and this seems to be a promising strategy to improve cytotoxicity of chemotherapy regimens.