International COPD Coalition Column: pulmonary rehabilitation-reaching out to our international community
The history of Pulmonary Rehabilitation (PR) dates back over decades and certain PR elements over centuries. The evidence of PR’s effectiveness has considerably strengthened and increased over the past forty years. During this time, the role and impact of PR has been firmly established and is now considered a key component in management of chronic respiratory disease. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) describes PR as an effective intervention for people with chronic obstructive pulmonary disease (COPD) with strong evidence for improved exercise capacity, health-related quality of life (HRQoL), symptoms of dyspnea, fatigue, anxiety and depression and reduction in healthcare utilization (see Table 1) (1). The 2006 American Thoracic Society (ATS)/European Respiratory Society (ERS) Statement on PR describes in detail the effectiveness of this intervention (2). The quality and grade of the evidence is outlined in the 2007 American College of Chest Physicians (ACCP)/American Association for Cardiovascular and Pulmonary Rehabilitation (AACVPR) Evidence Based Guidelines (3). Since these publications, there has been considerable growth in the knowledge of PR’s utility, effectiveness and scope (4). An updated review of the use and importance of PR will be described in the new ATS/ERS PR statement (due for publication in 2013) (5). This statement represents collaborative work of 46 international PR experts from a multidisciplinary background. PR offers a unique vehicle to coordinate care in the context of chronic lung disease and this statement will enhance the understanding of PR’s effectiveness and applications across international borders and various settings. It places PR within the concept of integrated care with the goal of improving access, quality, patient satisfaction and efficiency of this intervention. Key concepts discussed in this statement have been summarized in Table 2. This global perspective summarizes the important components of PR and describes its role and use in the US, Europe, Australia, New Zealand, Latin America and Japan.
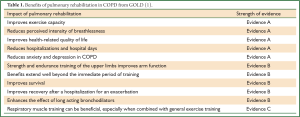
Full Table

Full Table
Overview of pulmonary rehabilitation
Effective PR begins with an individualized patient assessment, including medical history, physical exam and accurate diagnosis of the respiratory condition based on pulmonary function testing. Ascertaining the presence of comorbid conditions is important, particularly those that will impact an individual’s capacity to participate in exercise training. Baseline assessment should comprise measures of exercise capacity, symptoms and HRQoL (6). Individuals should be evaluated for hypoxemia at rest and during activity. Standardized questionnaires to measure symptoms and HRQoL are available in many languages and many are cost free and easy to use. In addition to laboratory-based tests of exercise capacity, there are valid, responsive and interpretable field-based tests that can be undertaken with minimal resources. Measures of exercise capacity, symptoms and HRQoL are made before and after PR and ideally during any long-term follow-up assessments to quantify the impact of PR and guide ongoing management.
Supervised aerobic exercise training is the foundation of effective PR. This usually involves walking and/or cycle-based exercise. Additional exercise prescription may include strength training and arm exercise. Strategies that serve to increase the likelihood of adopting a more active lifestyle following PR, such as the implementation of a home exercise program should be considered. There is also evidence to support the inclusion of disease-specific self-management strategies to address prevention, early identification and management of acute exacerbations of COPD (7). Other areas of importance include attending to nutritional status, counseling and optimization of supplemental oxygen systems in those who have been prescribed supplemental oxygen (Figure 1). Resources to assist healthcare providers establish a PR program are summarized in Table 3.
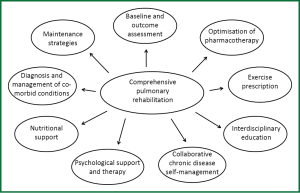
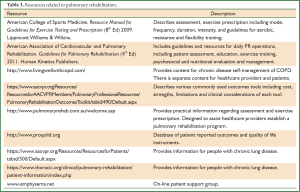
Full Table
Despite our increased understanding of the effect of PR, there are several areas that require further work. These include determining the impact of PR on daily physical activity and survival as well as effective strategies to optimize maintenance of benefit. The majority of evidence for PR pertains to people with COPD and there is a need for further research of its role in people with other chronic respiratory conditions.
Pulmonary rehabilitation in the United States of America
Formal PR in the US dates back to the 1960s with the foundation of PR established by Tom Petty, Mary Burns, Richard Casaburi, Andrew Ries and others. In 2010, Medicare national insurance for seniors began formal payment for PR in GOLD grades II-IV (moderate to very severe) COPD. This became law following an act of Congress making PR ‘the law of the land’ for those receiving Medicare benefits. There are now an estimated 1,000 PR programs in the US, with out-patient programs being the predominant model. Programs are typically 6-10 weeks in duration and usually provided by an interdisciplinary team that may include respiratory therapists, nurses, exercise physiologists, physical and occupational therapists, and headed by a physician medical director. National certification is available through the AACVPR that requires ongoing program evaluation of patient-centered outcomes, staff skill competency, and emergency preparedness www.aacvpr.org. Patient evaluation commonly includes field testing with a 6-minute walk test (6MWT), evaluation of dyspnea, HRQoL, and psychosocial symptoms. ATS established a PR Assembly in 2008 to further the representation and science of PR. A current priority is 2013 establishment of a national registry, led by the AACVPR, to aid programs in assessing quality and outcomes. Ongoing challenges include need for enhanced reimbursement due to variable insurance payment for services. Other challenges include low referral rates by some clinicians and limited access in rural and sparsely populated regions. An important resource for those with limited access to PR is a patient DVD entitled ‘Take Control of your COPD’ that details elements of PR, coping strategies and disease self-management techniques available at aacvpr.org in the patient resources section.
Pulmonary rehabilitation in Europe
Even though the exact number of PR programs in Europe and its content remains currently unknown, PR generally has been adopted as part on the integrated care of patients with chronic respiratory disease (8). However, large regional differences in the process and reimbursement of PR throughout Europe are to be expected (9). Indeed, PR programs in most parts of Eastern Europe are still rare. Therefore, ERS Group 01.02 specifically promotes PR and long-term care of patients with a chronic respiratory disease, including COPD.
On behalf of ERS Group 01.02, Drs. Donner and Howard wrote in 1992 the first international report on PR with a strong recommendation for its use in the management of patients with COPD (10). Next, multiple ERS members contributed to the 2006 and 2013 ATS/ERS Statements on Pulmonary Rehabilitation (2,5).
To date, ERS Group 01.02 still offers a unique opportunity to exchange information on the latest developments in PR. It surpasses the individual medical specialties by offering an international platform for interdisciplinary discussions on PR. Indeed, an interdisciplinary approach during a PR program is necessary to improve physical function, to adapt to the disease and to achieve health behavior change (5).
The clinical PR expertise in Europe is, at least is part, based on high-quality scientific research. Indeed, in the past decade, many original PR-related articles came from known European research groups, like Maastricht/Horn, the Netherlands (Emiel Wouters), Leuven, Belgium (Rik Gosselink), Leicester, UK (Sally Singh), Modena, Italy (Enrico Clini), Schoenau am Koenigssee (Klaus Kenn), and Athens, Greece (Ioannis Vogiatzis). Indeed, the rationale for and efficacy of PR during and directly following a hospital admission due to a COPD exacerbation came from European studies (11-16).
Pulmonary rehabilitation in Australia and New Zealand
National guidelines pertaining to the management of COPD highlight the importance of PR to optimize function (17). In Australia, approximately 240 PR programs are offered, with representation across all states and territories (18). In New Zealand, at least 21 PR programs are offered (19). In both countries, PR is most often funded by the government with the participant costs limited to travel and parking expenses. Programs are often provided in hospital out-patient departments and are about 8 weeks in duration (19,20). In Australia, PR programs are often coordinated by physiotherapists (20). Regarding program evaluation, in both countries, changes in exercise tolerance and HRQoL are commonly assessed using the 6MWT and disease-specific questionnaires such as the Chronic Respiratory Disease Questionnaire or the St George’s Respiratory Questionnaire, respectively (19,20). Very few programs have access to laboratory-based tests of exercise capacity. The most common components of a PR program are exercise training, education and self-management strategies (19,20). There is a strong research culture regarding PR in Australia with programs across several states contributing to the scientific literature in this area (21-23).
Despite the strong evidence for PR, in Australia, access to these programs is limited in areas of low population density. In an attempt to increase access to PR, several training programs have been developed with support from the Lung Foundation Australia. These include the ‘Breathe Easy, Walk Easy’ initiative as well as an on-line training program (24). The exercise training component described in these programs focuses on supervised high-intensity ground walking, which has been demonstrated to confer gains in walking endurance (25), indicating PR can be effective without access to exercise equipment (i.e. cycle ergometers, multi-gyms). Further, the Pulmonary Rehabilitation Toolkit was developed in conjunction with the Lung Foundation Australia and the Australian Physiotherapy Association as a resource that is freely available to those who are interested in establishing a PR program (see http://www.pulmonaryrehab.com.au/welcome.asp).
Following completion of PR, the benefits are often lost over a period of 12 to 18 months (3). Therefore, strategies to maintain the benefits of PR are of particular importance. Earlier work has shown that people who attend a community-based supervised exercise class once a week maintain the benefits of PR (26). Therefore, within Australia, there has been an increase in the number of community-based maintenance programs offered to people with chronic lung disease. The classes are often run in local community halls and are supervised by someone with appropriate knowledge of exercise for people with lung disease. They are offered either through the ‘Lungs in Action’ initiative of the Lung Foundation Australia, or Community Physiotherapy Services (in Western Australia). In most cases, there is a small cost to the participant to attend these classes. In some states, the scope of these community-based exercise classes is increasing such that selected individuals are now completing their initial PR in the community. This has the advantage of reducing travel and parking costs, which is an important barrier to attending PR programs offered in out-patient hospital departments.
Pulmonary rehabilitation in Latin America
In what concerns Latin America, countries in this region present wide variation in the structure of healthcare systems, as well as in the current development status of health-related care and research. Despite the fact that the prevalence of COPD in countries of the region ranges from 7.8% to as high as 19.7% (27), PR is a relatively recent field and for most of this short-lasting history there was little interaction among these countries concerning rehabilitation of patients with respiratory disorders. The creation of the Latin American Thoracic Association (ALAT) in the mid-90s (28) and its firm establishment since then has had a large impact in this interaction, which has evolved considerably.
The first PR program in Latin America was most likely the one in Santiago, Chile, which started in the mid-80s. Created in 1993, the program of the Federal University of São Paulo, Brazil has generated great impact in the region, and many centers which came afterwards were based on its experience. In 2000 there were approximately 28 PR centers in Latin America (29). There is no updated data on this topic, but this number has probably increased tremendously since it is currently estimated that Brazil alone has more than 100 PR programs. In recent years, Brazil and Argentina have led the region in terms of development of PR programs, although countries such as Mexico, Chile and Colombia have also presented considerable growth.
PR programs in Latin America vary considerably in structure, equipment, setting and the type of services and modalities provided. They range from small and simple programs comprising only medical (physician) care and exercise training, to highly skilled multidisciplinary rehabilitation centers. The vast majority of programs are outpatient-oriented, since Latin America has still little tradition in inpatient-oriented and home-based programs. Exercise training and education sessions are mostly held in groups, and assessment of benefits obtained from the program is commonly performed, especially concerning exercise capacity using the 6MWT (30). Most programs are connected to academic institutions, although private programs have steadily grown as well. As in many other world regions, reimbursement is a common and important issue, since no clear reimbursement policy is still available. Furthermore, the use of oxygen has been a challenge to many centers due to the “in development” socio-economic conditions that can be faced and the relatively limited access by patients with few resources. Moreover, for the same reasons, strategies to increase accessibility of patients to PR programs are imperative, especially in the public health system.
Pulmonary rehabilitation in Japan
One of the characteristic features of COPD in Japan is the impact of aging associated with general prolongation of the life span. The average life span in Japan is 80 years for men and 86 years for women, with the mean age of people with COPD being 77-79 years (31). The focus of PR in Japan includes supervising very old people with COPD in PR, as well as managing multiple difficulties in comprehension and adherence in these individuals. The second characteristic feature in people with COPD in Japan is malnourishment. Sugawara and colleagues found that people with COPD percent ideal body weight (%IBW) was 80-90%, with a mean body mass index (BMI) of approximately 18-20 kg/m2 (31). Therefore, PR provides active nutritional support, in addition to exercise therapy. The recent Cochrane systematic review summarized the effect of nutritional support in malnourished COPD patients in terms of weight gain, free fat mass, and respiratory muscle strength (32). The Cochrane review 2012 included two important trials from Japan and concluded that nutritional supplementation promotes significant weight gain among people with COPD, especially if malnourished, with significant improvement in fat-free mass index/fat-free mass, fat mass/fat mass index, 6-minute walk distance and a significant improvement in skinfold thickness (as measure of fat mass) for all patients. In those people who were malnourished, there was significant improvement in respiratory muscle strength (minimum inspiratory pressure and maximum expiratory pressure) and overall HRQoL. The Cochrane review supports the effectiveness of the model of PR used in many Japanese centers.
Conclusions
PR improves functional capacity, dyspnea, HRQoL and healthcare utilization in chronic lung disease, with the strongest evidence of effectiveness in persons with COPD. Formal PR is available in many developed countries and resources are available for establishing PR. Resources are also available for those unable to access PR. Future challenges include access to PR in sparsely populated areas and developing countries, and ensuring long term changes in physical activity and behavior following PR.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- From the Global Strategy for the Diagnosis Management and Preventation of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). Available online: http://www.goldcopd.org/
- Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006;173:1390-413. [PubMed]
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007;131:4S-42S. [PubMed]
- Goldstein RS, Hill K, Brooks D, et al. Pulmonary rehabilitation: a review of the recent literature. Chest 2012;142:738-49. [PubMed]
- Spruit MA, Singh S, Garvey C, et al. An official American Thoracic Soceity/European Respiratory Society Statement: Key concepts and advances in pulmonary rehabilitation - an executive summary. Am J Respir Crit Care Med 2013. [Epub ahead of print].
- Jenkins S, Hill K, Cecins NM. State of the art: how to set up a pulmonary rehabilitation program. Respirology 2010;15:1157-73. [PubMed]
- Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med 2003;163:585-91. [PubMed]
- Nici L, ZuWallack R, American Thoracic Society Subcommittee on Integrated Care of the COPD Patient. An official American Thoracic Society workshop report: the Integrated Care of The COPD Patient. Proc Am Thorac Soc 2012;9:9-18. [PubMed]
- Yohannes AM, Connolly MJ. Pulmonary rehabilitation programmes in the UK: a national representative survey. Clin Rehabil 2004;18:444-9. [PubMed]
- Donner CF, Howard P. Pulmonary rehabilitation in chronic obstructive pulmonary disease (COPD) with recommendations for its use. Report of the European Respiratory Society Rehabilitation and Chronic Care Scientific Group (S.E.P.C.R. Rehabilitation Working Group). Eur Respir J 1992;5:266-75. [PubMed]
- Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003;58:752-6. [PubMed]
- Man WD, Polkey MI, Donaldson N, et al. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 2004;329:1209. [PubMed]
- Pitta F, Troosters T, Probst VS, et al. Physical activity and hospitalization for exacerbation of COPD. Chest 2006;129:536-44. [PubMed]
- Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010;65:423-8. [PubMed]
- Troosters T, Probst VS, Crul T, et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;181:1072-7. [PubMed]
- Puhan MA, Gimeno-Santos E, Scharplatz M, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011;(10):CD005305. [PubMed]
- The COPD-X plan: Australia and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease V2.30. 2011. Available online: http://www.lungfoundation.com.au/professional-resources/guidelines/copd-x-plan
- Lung Foundation Australia. Available online: http://www.lungfoundation.com.au/professional-resources/pulmonary-rehabilitation/
- Levack WM, Weatherall M, Reeve JC, et al. Uptake of pulmonary rehabilitation in New Zealand by people with chronic obstructive pulmonary disease in 2009. N Z Med J 2012;125:23-33. [PubMed]
- Johnston CL, Maxwell LJ, Alison JA. Pulmonary rehabilitation in Australia: a national survey. Physiotherapy 2011;97:284-90. [PubMed]
- Jenkins S, Čečins N. Six-minute walk test: observed adverse events and oxygen desaturation in a large cohort of patients with chronic lung disease. Intern Med J 2011;41:416-22. [PubMed]
- Holland AE, Hill CJ, Glaspole I, et al. Predictors of benefit following pulmonary rehabilitation for interstitial lung disease. Respir Med 2012;106:429-35. [PubMed]
- McNamara RJ, McKeough ZJ, McKenzie DK, et al. Water-based exercise in copd with physical co-morbidities: a randomised controlled trial. Eur Respir J 2013;41:1284-91. [PubMed]
- Johnston CL, Maxwell LJ, Boyle E, et al. Improving chronic lung disease management in rural and remote Australia: the Breathe Easy Walk Easy programme. Respirology 2013;18:161-9. [PubMed]
- Leung RW, Alison JA, McKeough ZJ, et al. Ground walk training improves functional exercise capacity more than cycle training in people with chronic obstructive pulmonary disease (COPD): a randomised trial. J Physiother 2010;56:105-12. [PubMed]
- Cockram J, Cecins N, Jenkins S. Maintaining exercise capacity and quality of life following pulmonary rehabilitation. Respirology 2006;11:98-104. [PubMed]
- Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet 2005;366:1875-81. [PubMed]
- Asociacion Latinoamericana de Torax. Available online: http://www.alatorax.org
- Jardim JR, Camelier AA, Miki, D. The Latin American Persepctive. In: Pulmonary Rehabilitation - Guidelines to Success. 3rd ed. USA: Lippincott Williams & Wilkins, 2000:661-6.
- Tramontini MR, Mayer AF, Cardosoa F, et al. Variability in walk test conditions in pulmonary rehabilitation programs in Latin America and on the Iberian peninsula. Arch Bronconeumol 2005;41:667-78. [PubMed]
- Sugawara K, Takahashi H, Kasai C, et al. Effects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPD. Respir Med 2010;104:1883-9. [PubMed]
- Ferreira IM, Brooks D, White J, et al. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;12:CD000998. [PubMed]


