A novel hybrid technique for localization of subcentimeter lung nodules
Introduction
In recent years, the development of computed tomography (CT) technique and the expansion of low-dose CT screening programs have led to rapidly increasing number of newly-diagnosed patients with subcentimeter lung nodules. It is noteworthy that lung nodules >5 mm in size carry a high risk of malignancy, highlighting the necessity of early intervention (1). Either for diagnostic or therapeutic purposes, surgery plays a crucial role for these patients, and (VATS) is viewed as an ideal approach by most surgeons. However, it is highly challenging to locate these tiny lesions through the small incisions of VATS, especially when the lesions are nonpalpable pure ground-glass nodules (GGNs) and deeply situated in the lung tissue. For some cases, conversion to open thoracotomy is likely unavoidable (2). Therefore, preoperative localization of small lung nodule has been widely accepted to as a solution and proved to be useful.
To date, a variety of localization techniques have been established, such as hook-wire or microcoil labelling, intraoperative ultrasound scan, methylene blue staining and radio-guided detection. Among them, CT-guided hook-wire or microcoil techniques are preferable due to higher sensitivity, less operator dependence and lower complication rate (3-5). Nevertheless, dislodgment of the wire or coil after the lung collapses in the surgery remains a disturbing problem and leads to a relatively higher failure rate of marking (6). In the current study, we developed a novel hybrid technique, which combines induced controllable pneumothorax and CT-guided microcoil marking, to reduce or even eliminate the risk of dislodgment.
Methods
Between May 2015 and March 2016, 35 patients with 37 subcentimeter lung GGNs who underwent preoperative CT-guided microcoil marking and VATS resections at our institution were retrospectively reviewed. Only subpleural lesions, presumed impalpable, with a maximum distance to the pleural surface of 30mm were included. Lesions were incidentally found in 33 patients while they underwent health examination or were being examined for other medical reasons. In 2 patients with a clinical history of malignancy, the lesions were found during follow-up examination. No preoperative pathologic diagnoses were obtained, either by fiberoptic bronchoscopy (FOB) or by CT-guided needle lung biopsies.
Preoperative marking procedure
The preoperative CT scan was evaluated in an interdisciplinary setting, including the surgeons (X.P, L.X, J.D) and the radiologist (J.C), to determine whether the patient required the CT-guided microcoil marking procedure. All patients signed a written informed content and underwent the procedure on the same day of surgery or the day before. First, a CT scan was performed to locate the lesions and the needle access route was planned before inducing minor pneumothorax. The position of patient on the CT table depended on the shortest distance between the skin and the lesion and simultaneously allowed a safe access with the needle. After local anesthesia, a 21-gauge, 150-mm-long Chiba needle (Cook, Bloomington, IN, USA) was utilized, under CT guidance, to inject 200–300 mL air into the thoracic cavity. After the lung collapsed slightly, the same needle was loaded with the fiber-coated platinum microcoil (Vortex-18, Diamond Shape; Boston Scientific, Cork, Ireland), and then was advanced to the lung parenchyma adjacent to the target lesion (≤1.5 cm). A 40-cm-length 0.018-inch guidewire (Angiodynamics Vaxcel Mini Stick Kit, Glen Falls, NY, USA), which had been pre-marked for the length of the microcoil segment intended to be anchored in the lung parenchyma, was then gradually inserted into the needle, pushing the head segment of the microcoil into the lung parenchyma near the lesion, while the tail segment was left on the pleural surface for subsequent visual identification during VATS (7,8). A final CT scan was performed to confirm the exact location of the microcoil and no chest tube was placed if the patient had no respiratory symptoms.
Surgical procedures
VATS was performed under general anesthesia using single lung ventilation via a double-lumen endobronchial tube. Two or three incisions were made, one for the thoracoscope (usually located at the midaxillary line of the 8th intercostal space), the other one or two incisions (usually located at the anterior axillary line of the 4th or 5th intercostal space and the scapular line of the 5th or 6th intercostal space) for the resection using an endoscopic linear stapler. When the marking microcoil was visually identified, finger palpation was first applied to confirm the location of the lesions. Then a wedge resection was performed if the lesions situated adjacent to the pleural surface (<2 cm). Otherwise, a segmentectomy was conducted. Excised specimen included the target lesion, the surrounding lung parenchyma and the marking microcoil, which was instantly sent for intraoperative frozen-section examination. VATS lobectomy and lymphadenectomy was performed subsequently in wedge resection cases when required, according to pathology analysis. While in the segmentectomy cases, lymph node sampling was conducted subsequently when pathology analysis indicated necessary.
Results
Between May 2015 and March 2016, 35 patients with 37 subcentimeter lung GGNs underwent preoperative CT-guided microcoil marking procedure and VATS resection at our institution. There were 15 men and 20 women, and the mean age was 51.2±7.6 years (range, 39–62 years). The size of the lesions at the CT scan ranged between 6 and 10 mm (mean size, 7.1±2.7 mm). All 37 lung lesions (24 pure GGNs, 13 mixed GGNs) were successfully marked through CT-guided microcoil marking procedure preoperatively. No major complications were observed during the procedure, which took a mean duration of 32.7 minutes per patient (range, 16–49 minutes). The average time for inducing pneumothorax was 5.7minutes, and the mean distance between the lesion and the pleural surface was 13.4±8.1 mm. None of the 33 patients with single lesion required chest tube insertion for the minor pneumothorax, neither did the two cases with double lesions. Minor bleeding within the lung tissue, indicated by small areas of consolidation on CT scan, was found in 12 patients (34.3%), with no hemoptysis occurring. The tails of the microcoils were successfully exposed on the pleural surface in 32 cases (91.4%).
Among the 35 patients, 29 (82.9%) underwent the marking procedure on the day before surgery, while the other 6 (17.1%) had their lesions marked on the same day of surgery. The time to perform the VATS resection ranged from 2.3 to 27.6 hours (mean 18.1±2.2 hours). In All 35 cases, surgical resection was accomplished through VATS, with no dislodgment of microcoil observed in the surgery. Twenty-seven patients underwent VATS wedge resection first (Figure 1), among whom 8 patients subsequently received VATS lobecotomy and lymphadenectomy as the lesions were revealed to be invasive adenocarcinoma by intraoperative pathologic analysis. The other 8 patients underwent VATS segmentectomy due to deeply situated lesions, among whom 2 cases required lymph node sampling when pathologic analysis indicated minimally invasive adenocarcinoma (MIA). In the 2 cases with double lesions, sequential wedge resections were performed as frozen-section examination demonstrated atypical adenomatous hyperplasia (AAH) or adenocarcinoma in situ (AIS).
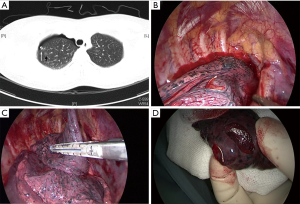
There were no perioperative or postoperative mortality related to the surgical resection, and only one minor hemothorax was incidentally found in the VATS resection, in which case the bleeding was caused by puncture injury of the lung related to the marking procedure. The patient required no hemostasis and recovered with no further incident. Hospital stay ranged from 4 to 8 days (mean 4.9±1.6 days).
Postoperative analysis of the surgical specimens from 31 patients demonstrated the presence of malignancy in 33 lesions, including 6 AIS, 17 MIA, 8 invasive adenocarcinoma and 2 metastatic adenocarcinoma. In 2 cases, the lesions were diagnosed as AAH, while benign origin was conformed in the lesions of the remaining 2 patients. All key data were summarized in Table 1.
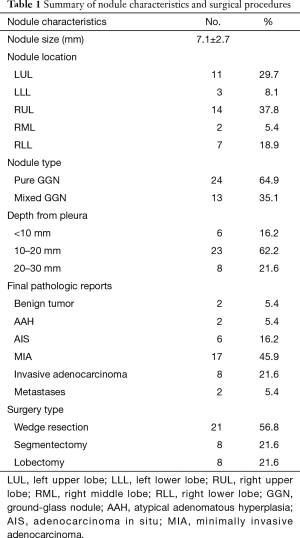
Full table
Discussion
Locating a subcentimeter lesion in the lung parenchyma intraoperatively has usually been a time-consuming and skill-demanding task for a thoracic surgeon, especially in the VATS cases. With preoperative localization techniques, however, surgeons will feel more confident in resecting the target lesions and surgical procedures can be significantly expedited (9). A variety of methods has been developed thus far, and each of them has its own advantages and drawbacks.
Although methylene blue staining of the lesions yielded high sensitivity (10,11), the spread of the colored substances into surrounding lung parenchyma and the possibility of color fading remain to be problematic (12). Radio-guided detection was also favored for its high sensitivity (13,14), whereas the need of special instruments and radiation-related hazards limit its adoption by many institutions and centers. As for intraoperative ultrasound detection, it has been reported to be efficient and non-invasive, but operator-dependence and disappointing performance in emphysema lung parenchyma undermine its popularity in clinical practice (15,16).
CT-guided marking of lung nodule using hook-wire or microcoil has been repeatedly reported in the literature and proved to be practicable with high sensitivity (3-5). However, dislodgment of the wires or coils after lung collapses is a frustrating problem and was reported to occur in up to 47% of cases who underwent the hook-wire marking procedure (17), which remarkably dampens the outcome of marking. As for the microcoil marking technique, the dislodgement rate was comparatively lower reported in the literature (8,18), especially when the coil is used only to label the target nodule but not the surface of the lung (19). But for beginners within the learning curve or in cases with unconsolidated GGNs, the dislodgement of microcoil remains a headache. The dislodged coils either hang onto the chest wall or fall into the corners of thoracic cavity. We speculated that the greater grasping force upon the microcoil applying from the chest wall than the one from the lung parenchyma might be the ‘culprit’ causing the dislodgement. In the first scenario, the microcoil is ‘captured’ by the chest wall in the ‘tug of war’ with the lung parenchyma when the lung collapses. While in the second one, it is possible that the microcoil is ‘clutched’ by the chest wall in the first place, but then falls off due to the gravity of itself. Therefore, eliminating the grasping force from the chest wall may be a feasible approach to avoid the dislodgement. To this end, we introduced minor pneumothorax before placing the microcoil in the marking procedure, so that the chest wall was excluded from the 'tug of war' and the microcoil was ‘clutched’ only by the surrounding lung parenchyma, which resulted in a firm anchorage and a zero rate of dislodgement.
One caveat of our technique is that the extent of pneumothorax should be delicately adjusted in the cases with pure ground-glass opacity (GGO) lesions located very closely to the pleural surface. Collapsed lung tissue may obscure these low-density lesions on CT scan, which occurred in 3 cases of our series, significantly increasing the risk of marking failure and the procedure time. Injecting less air or withdrawing some air when lung deflated too much will be helpful. On the other hand, hindrance from ribs or other parietal structure might render the nodules situated just below the pleural surface more difficult to label directly than those located deeper in the lung parenchyma. Our technique, which creates some distance between the lung and the chest wall by inducing pneumothorax before marking, might be an available solution in some cases.
Pleural adhesion would surely be a hindrance when applying this technique, as it can significantly increase the difficulty of introducing pneumothorax and preoperative CT scan cannot detect it in most cases. Although we did not encounter such cases in our series, great care should be taken to avoid the risk of injecting the air into lung tissue by mistake when inducing pneumothorax. If the attempt of inducing pneumothorax fails initially, it is recommended to place the microcoil directly and immediately without more attempts. To reduce the possibility of dislodgement, implanting one microcoil of larger size or two microcoils might be helpful as our early experience of preoperative marking procedures showed (date not included).
Several authors reported that the spiral-wire or microcoil technique allows the traction of lung lesions towards the chest wall, therefore making the placement of linear staples more accurate and expeditious (20). It was recommended to place the wire or coil right into the solid nodule or more than 2 cm deep into the lung tissue for more stable anchorage (21). However, our experience demonstrated that this kind of manipulation might be unsuitable for GGN lesion, as it may dramatically increase the chance of dislodgment due to the comparatively soft and loose composition of these lesions.
Conclusions
In summary, this study suggests that the hybrid technique, combining induced minor pneumothorax and CT-guided microcoil marking procedure, is a safe, effective and reliable approach for preoperative localization of small lung GGN. Compared with other marking-only methods using wires or coils, it significantly lowers the risk of dislodgement.
Acknowledgement
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Zhongshan Hospital Ethics Committee and written informed consent was obtained from all patients.
References
- Henschke CI, Yankelevitz DF, Naidich DP, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology 2004;231:164-8. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Torre M, Ferraroli GM, Vanzulli A, et al. A new safe and stable spiral wire needle for thoracoscopic resection of lung nodules. Chest 2004;125:2289-93. [Crossref] [PubMed]
- Paci M, Annessi V, Giovanardi F, et al. Preoperative localization of indeterminate pulmonary nodules before videothoracoscopic resection. Surg Endosc 2002;16:509-11. [Crossref] [PubMed]
- Wicky S, Dusmet M, Doenz F, et al. Computed tomographyguided localization of small lung nodules before videoassisted resection: experience with an efficient hook-wire system. J Thorac Cardiovasc Surg 2002;124:401-3. [Crossref] [PubMed]
- Bernard A. Resection of pulmonary nodules using video-assisted thoracic surgery. The Thorax Group. Ann Thorac Surg 1996;61:202-4. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Powell TI, Jangra D, Clifton JC, et al. Peripheral lung nodules: fluoroscopically guided video-assisted thoracoscopic resection after computed tomography-guided localization using platinum microcoils. Ann Surg 2004;240:481-8. [Crossref] [PubMed]
- Sui X, Zhao H, Yang F, et al. Computed tomography guided microcoil localization for pulmonary small nodules and ground-glass opacity prior to thoracoscopic resection. J Thorac Dis 2015;7:1580-7. [PubMed]
- McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg 2002;37:1729-31. [Crossref] [PubMed]
- Partrick DA, Bensard DD, Teitelbaum DH, et al. Successful thoracoscopic lung biopsy in children utilizing preoperative CT-guided localization. J Pediatr Surg 2002;37:970-3. [Crossref] [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref] [PubMed]
- Burdine J, Joyce LD, Plunkett MB, et al. Feasibilily and value of video-assisted thoracoscopic surgery wedge excision of small pulmonary nodules in patients with malignancy. Chest 2002;122:1467-70. [Crossref] [PubMed]
- Chella A, Lucchi M, Ambrogi MC, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg 2000;18:17-21. [Crossref] [PubMed]
- Matsumoto S, Hirata T, Ogawa E, et al. Ultrasonographic evaluation of small nodules in the peripheral lung during video-assisted thoracic surgery (VATS). Eur J Cardiothorac Surg 2004;26:469-73. [Crossref] [PubMed]
- Santambrogio R, Montorsi M, Bianchi P, et al. Intraoperative ultrasound during thoracoscopic procedures for solitary pulmonary nodules. Ann Thorac Surg 1999;68:218-22. [Crossref] [PubMed]
- Gonfiotti A, Davini F, Vaggelli L, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: hookwire versus radio-guided surgery. Eur J Cardiothorac Surg 2007;32:843-7. [Crossref] [PubMed]
- Su TH, Fan YF, Jin L, He W, et al. CT-guided localization of small pulmonary nodules using adjacent microcoil implantation prior to video-assisted thoracoscopic surgical resection. Eur Radiol 2015;25:2627-33. [Crossref] [PubMed]
- Kha LC, Hanneman K, Donahoe L, Chung T, et al. Safety and Efficacy of Modified Preoperative Lung Nodule Microcoil Localization Without Pleural Marking: A Pilot Study. J Thorac Imaging 2016;31:15-22. [Crossref] [PubMed]
- Eichfeld U, Dietrich A, Ott R, Kloeppel R. Video-assisted thoracoscopic surgery for pulmonary nodules after computed tomography-guided marking with a spiral wire. Ann Thorac Surg 2005;79:313-6. [Crossref] [PubMed]
- Partik BL, Leung AN, Muller MR, et al. Using a dedicated lung-marker system for localization of pulmonary nodules before thoracoscopic surgery. AJR Am J Roentgenol 2003;180:805-9. [Crossref] [PubMed]


